Brain Biology and Chemistry

Presentation
« Brain Biology & Chemistry » team belongs to the « Lille Neuroscience & Cognition » research center and is located in Lille, France.
The team is composed of an Inserm researcher, eleven professors and associate professors, two hospital practitioners. It benefits from the technical support of seven people and welcomes many students: from master degree, PhD students to post-doc fellows.
Our multidisciplinary team brings together chemists, biologists and spectroscopists.
- STAFF
- RESEARCH INTEREST
- PUBLICATIONS
Associate Professors/Professors – University-Associated
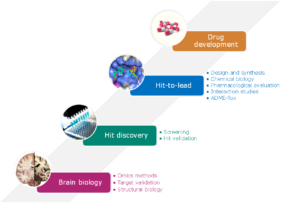
Neurodegenerative diseases are characterized by common biological hallmarks such as inflammation, aggregation of altered proteins, modification of cell and vesicular trafficking, cellular (or neuronal) degeneration leading to cognitive impairments. We want to design and caracterize new molecules active on these hallmarks. To do this, we have identified targets of interest on which we are focusing our efforts and we will continue to define relevant targets using Omics approaches on patient samples. New emerging targets are under studying.
More specifically, we are studying targets linked to defects in cell trafficking and their involvement in protein homeostasis, such as LRRK2 or alpha synuclein for the treatment of Parkinson's disease, the Hippo pathway in Huntington's disease or sigma-1 for the treatment of Multiple Sclerosis. Then we develop the evaluation tests (enzymatic, biophysical or cellular methods) and test collections of compounds. Molecular modeling and medicinal chemistry allow us to optimize the compounds and go from hits to leads. Of greatest interest in neurodegenerative diseases is the study of modulators of protein-protein interactions. For instance, we are developing TEAD modulators or photo-switchable compounds capable of destroying protein aggregates. Autophagy modulating compounds are being studied for the treatment of Alzheimer's disease. One of these compounds is now in phase 2. Pharmacological studies and ADME Tox provide drug candidates.
Publications
2022
Aidibi Y, Beaujean P, Quertinmont J, Stiennon J, Hodée M, Leriche P, Berthet J, Delbaere S, Champagne B, Sanguinet L.
A molecular loaded dice: When the π conjugation breaks the statistical addressability of an octastate multimodal molecular switch. Dyes Pigments 2022, 202, 110270
Bdair H, Singleton TA, Ross K, Jolly D, Kang MS, Aliaga A, Tuznik M, Kaur T, Yous S, Soucy JP, Massarweh G, Scott PJH, Koeppe R, Spadoni G, Bedini A, Rudko DA, Gobbi G, Benkelfat C, Rosa-Neto P, Brooks AF, Kostikov A.
Radiosynthesis and In Vivo Evaluation of Four Positron Emission Tomography Tracer Candidates for Imaging of Melatonin Receptors, ACS Chem Neurosci 2022, 13(9), 1382-1394![]()
Blaise AS, Cuvelier E, Carrière N, Devos D, Moreau F, Defebvre L, Mutez E.
Use of levodopa-carbidopa intestinal gel to treat patients with multiple system atrophy, Parkinsonism Relat Disord 2022, 100, 41-44![]()
Bolteau R, Duroux R, Laversin A, Vreulz B, Shiriaeva A, Stauch B, Han G-W, Cherezov V, Renault N, Barczyk A, Ravez S, Coevoet M, Melnyk P, Liberelle M, Yous S.
High ligand efficiency quinazoline compounds as novel A2A adenosine receptor antagonists. Eur. J Med Chem, 2022, 241, 114620![]()
Cogo S, Y Ho F, Tosoni E, E Tomkins J, Tessari I, Lannotta L, J Montine T, Manzoni C, A Lewis P, Bubacco L, Chartier Harlin M-C, Taymans J-M, Kortholt A, Nichols R.J, Cendron L, Civiero L, Greggio E.
The Roc domain of LRRK2 as a hub for protein-protein interactions: a focus on PAK6 and its impact on RAB phosphorylation. Brain Res 2022, 1778, 147781![]()
Coku I, Mutez E, Eddarkaoui S, Carrier S, Marchand A, Deldycke C, Goveas L, Baille G, Tir M, Magnez R, Thuru X, Vermeersch G, Vandenberghe W, Buée L, Defebvre L, Sablonnière B, Chartier-Harlin M-C, Taymans J-M, Huin V.
Functional Analyses of Two Novel LRRK2 Pathogenic Variants in Familial Parkinson's Disease. Mov Disord 2022, 37(8), 1761 ![]()
Domenighetti C, Douillard V, Sugier P-E, Ashwin Ashok Kumar Sreelatha, Schulte C, Grover S, May P, R. Bobbili D, Radivojkov-Blagojevic M, Lichtner P, B. Singleton A, G. Hernandez D, Edsall C, Gourraud P-A, Mellick D, Zimprich A, Pirker W, Rogaeva E, Lang A, Koks S, Taba P, Lesage S, Brice A, Corvol J-C, Chartier-Harlin M-C, Mutez E, Brockmann K, B. Deutschländer A, M. Hadjigeorgiou G, Dardiotis E, Stefanis L, Maria Simitsi A, Maria Valente E, Petrucci S, Duga S, Straniero L, Zecchinelli A, Pezzoli G, Brighina L, Ferrarese C, Annesi G, Quattrone A, Gagliardi M, Matsuo H, Nakayama A, Hattori N, Nishioka K, Ju Chung S, Joong Kim Y, Kolber P, PC van de Warrenburg B, R. Bloem B, Aasly J,Toft M, Pihlstrøm L, Correia Guedes L, J.Ferreira J, Bardien S, Carr J, Tolosa E, Ezquerra M, Pastor P, Diez-Fairen M, Wirdefeldt K, L. Pedersen N, Ran C, C. Belin A, Puschmann A, Ygland Rödström E, E. Clarke C, E.Morrison K, Tan M, Krainc D, F. Burbulla L, J. Farrer M, Krüger R, Gasser T, Sharma M, Vince N, Elbaz A. On behalf of the Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson’s Disease (Courage-PD) consortium.
The Interaction between HLA-DRB1 and Smocking in Parkinson’s
Mov. Disord 2022, 37(9), 1929-1937![]()
Domenighetti C, Sugier P-E, Ashok Kumar Sreelatha A, Schulte C, Grover S, Mohamed O, Portugal B, May P, Bobbili D-R, Radivojkov-Blagojevic M, Lichtner P, Singleton A-B, Hernandez D-G, Edsall C, Mellick G-D, Zimprich A, Pirker W, Rogaeva E, Lang A-E, Koks S, Taba P, Lesage S, Brice A, Corvol JC, Chartier-Harlin M-C, Mutez E, Brockmann K, Deutschländer A-B, Hadjigeorgiou G-M, Dardiotis E, Stefanis L, Simitsi A-M, Valente E-M, Petrucci S, Duga S, Straniero L, Zecchinelli A, Pezzoli G, Brighina L, Ferrarese C, Annesi G, Quattrone A, Gagliardi M, Matsuo H, Kawamura Y, Hattori N, Nishioka K, Chung SJ, Kim Y-J, Kolber P, van de Warrenburg BPC, Bloem B-R, Aasly J, Toft M, Pihlstrøm L, Correia Guedes L, Ferreira J-J, Bardien S, Carr J, Tolosa E, Ezquerra M, Pastor P, Diez-Fairen M, Wirdefeldt K, Pedersen N-L, Ran C, Belin A-C, Puschmann A, Hellberg C, Clarke CE, Morrison K-E, Tan M, Krainc D, Burbulla L-F, Farrer M-J, Krüger R, Gasser T, Sharma M, Elbaz A and Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson's Disease (Courage-PD) Consortium.
Dairy Intake and Parkinson's Disease: A Mendelian Randomization Study. Mov Disord 2022, 37(4):857-864![]()
Domenighetti C, Sugier PE, Sreelatha AAK, Schulte C, Grover S, Mohamed O, Portugal B, May P, Bobbili DR, Radivojkov-Blagojevic M, Lichtner P, Singleton AB, Hernandez DG, Edsall C, Mellick GD, Zimprich A, Pirker W, Rogaeva E, Lang AE, Koks S, Taba P, Lesage S, Brice A, Corvol JC, Chartier-Harlin MC, Mutez E, Brockmann K, Deutschländer AB, Hadjigeorgiou GM, Dardiotis E, Stefanis L, Simitsi AM, Valente EM, Petrucci S, Duga S, Straniero L, Zecchinelli A, Pezzoli G, Brighina L, Ferrarese C, Annesi G, Quattrone A, Gagliardi M, Matsuo H, Kawamura Y, Hattori N, Nishioka K, Chung SJ, Kim YJ, Kolber P, van de Warrenburg BP, Bloem BR, Aasly J, Toft M, Pihlstrøm L, Guedes LC, Ferreira JJ, Bardien S, Carr J, Tolosa E, Ezquerra M, Pastor P, Diez-Fairen M, Wirdefeldt K, Pedersen NL, Ran C, Belin AC, Puschmann A, Hellberg C, Clarke CE, Morrison KE, Tan M, Krainc D, Burbulla LF, Farrer MJ, Krüger R, Gasser T, Sharma M, Elbaz A, Comprehensive Unbiaised Risk Factor Assessment for Genetics and Environment in Parkinson’s Disease (Courage-PD) consortium.
Mendelian Randomisation Study of Smoking, Alcohol, and Coffee Drinking in Relation to Parkinson's Disease. J Parkinsons Dis 2022, 12(1):267-282![]()
Ekmen A, Meneret A, Valabregue R, Beranger B, Worbe Y, Lamy JC, Mehdi S, Herve A, Adanyeguh I, Temiz G, Damier P, Gras D, Roubertie A, Piard J, Navarro V, Mutez E, Riant F, Welniarz Q, Vidailhet M, Lehericy S, Meunier S, Gallea C, Roze E.
Cerebellum Dysfunction in Patients With PRRT2-Related Paroxysmal Dyskinesia. Neurology 2022, 98(10), e1077-e1089![]()
Fernández B, Chittoor-Vinod VG, Kluss JH, Kelly K, Bryant N, Nguyen APT, Bukhari SA, Smith N, Lara Ordóñez AJ, Fdez E, Chartier-Harlin MC, Montine TJ, Wilson MA, Moore DJ, West AB, Cookson MR, Nichols RJ, Hilfiker S.
Evaluation of Current Methods to Detect Cellular Leucine-Rich Repeat Kinase 2 (LRRK2) Kinase Activity. J Parkinsons Dis 2022 , 15(5), 1423-1447 ![]()
Grover S, Ashwin AKS, Pihlstrom L, Domenighetti C, Schulte C, Sugier P-E, Radivojkov-Blagojevic M, Lichtner P, Mohamed O, Portugal B, Landoulsi Z, May P, Bobbili D, Edsall C, Bartusch F, Hanussek M, Krüger J, Hernandez D-G, Blauwendraat C, Mellick G-D, Zimprich A, Pirker W, Tan M, Rogaeva E, Lang A, Koks S, Taba P, Lesage S, Brice A, Corvol J-C, Chartier-Harlin M-C, Mutez E, Brockmann K, Deutschländer A-B, Hadjigeorgiou G-M, Dardiotis E, Stefanis L, Simitsi A-M, Valente E-M, Petrucci S, Straniero L, Zecchinelli A, Pezzoli G, Brighina L, Ferrarese C, Annesi G, Quattrone A, Gagliardi M, Burbulla L-F, Matsuo H, Kawamura Y, Hattori N, Nishioka K, Chung S-J, Kim Y-J, Pavelka L, van de Warrenburg B-P, Bloem B-R, Singleton A-B, Aasly J, Toft M, Guedes L-C, Ferreira J-J, Bardien S, Carr J, Tolosa E, Ezquerra M, Pastor P, Diez-Fairen M, Wirdefeldt K, Pedersen N-L, Ran C, Belin A-C, Puschmann A, Hellberg C, Clarke CE, Morrison KE, Krainc D, Farrer MJ, Kruger R, Elbaz A, Gasser T, Sharma M; and the Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson’s Disease (COURAGE-PD) consortium.
Genome-wide Association and Meta-analysis of Age-at-Onset in Parkinson Disease: Evidence From COURAGE-PD Consortium. Neurology 2022, 99(7), e698-e710 ![]()
Hernández S, Feracci M, De Jesus C-T, El Kazzi P, Kaci R, Garlatti L, Mondielli C, Bailly F, Cotelle P, Touret F, de Lamballerie X, Coutard B, Decroly E, Canard B, Ferron F, Alvarez K.
Identification of potent inhibitors of arenavirus and SARS-CoV-2 exoribonucleases by fluorescence polarization assay. Antivir. Res 2022, 204, 105364![]()
Kreisler A, Djelad S, Simonin C, Baille G, Mutez E, Degardin A, Defebvre L, Labreuche J, Cailliau E, Duhamel A.
Does ultrasound-guidance improve the outcome of botulinum toxin injections in cervical dystonia? Rev Neurol (Paris) 2022, 178(6), 591-602![]()
Legros C, Yous S, Boutin JA.
Alternative Ligands at Melatonin Receptors. Methods Mol Biol 2022, 2550, 151-162![]()
Liberelle M, Toulotte F, Renault N, Gelin M, Allemand F, Melnyk P, Guichou J-F, Cotelle P.
Towards the design of selective ligands of TEAD C-terminal domain, J. Med. Chem. 2022, 65, 5926-5940
Liu H, Dehestani M, Blauwendraat C, Makarious M-B, Leonard H, Kim J-J, Schulte C, Noyce A, Jacobs B-M, Foote I, Sharma M; International Parkinson's Disease Genomics Consortium; Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson's Disease Consortium, Nalls M, Singleton A, Gasser T, Bandres-Ciga S. Ann.
Polygenic Resilience Modulates the Penetrance of Parkinson Disease Genetic Risk Factors. Neurol 2022, 92, 270-278![]()
Marchand A, Sarchione A, Athanasopoulos P-S, Roy H-B, Goveas L, Magnez R, Drouyer M, Emanuele M, Ho F-Y, Liberelle M, Melnyk P, Lebègue N, Thuru X, Nichols R-J, Greggio E, Kortholt A, Galli T, Chartier-Harlin M-C, Taymans J-M.
A Phosphosite Mutant Approach on LRRK2 Links Phosphorylation and Dephosphorylation to Protective and Deleterious Markers, Respectively. Cells. 2022 Mar 17;11(6), 1018![]()
Marteau R, Ravez S, Mazhari D, Bouchaoui H, Porte K, Devedjian JC, Melnyk P, Devos D, Frederick R, El Bakali J.
Repositioning of FDA-Approved Antifungal Agents to interrogate Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4) inhibitors in Ferroptosis. Biochem. Pharmacol, 2022, 204, 115239![]()
Mésangeau C, Carato P, Renault N, Coevoet M, Barczyk A, Buée L, Sergeant N, Melnyk P.
Discovery of Compounds that Selectively Repress the Amyloidogenic Processing of the Amyloid Precursor Protein : Design, Synthesis and Pharmacological Evaluation of Diphenylpyrazoles. Int J Mol Sci 2022, 23(21), 13111![]()
Oxombre B, Madouri F, Journé A-S, Ravez S, Woitrain E, Odou P, Duhal N, Ninni S, Montaigne D, Delhem N, Vermersch P, Melnyk P.
Safe and efficient sigma1 ligand: a potential drug candidate for Multiple Sclerosis. Int. J. Mol. Sci., 2022, 23(19), 11893![]()
Petitgas P, Tattevin P, Mailles A, Fillâtre P, Stahl JP, ENCEIF scientific committee investigators group.
Infectious encephalitis in elderly patients: a prospective multicentre observational study in France 2016-2019. Infection 2022, [in press]![]()
Quenon C, Hennebelle T, Butaud J-F, Ho R, Samaillie J, Neut C, Lehartel T, Rivière C, Siah A, Bonneau N, Sahpaz S, Anthérieu S, Lebègue N, Raharivelomanana P, Roumy V.
Antimicrobial Properties of Compounds Isolated from Syzygium malaccense (L.) Merr. and L.M. Perry and Medicinal Plants Used in French Polynesia. Life 2022, 12(5), 733![]()
Rapetti-Mauss R, Nigri J, Bérenguier C, Finetti P, Simha Tubiana S, Labrum B, Allégrini B, Pellissier B, Bousquet C, Dusetti N, Bertucci F, Guizouarn H, Melnyk P, Borgese F, Tomasini R, Soriani O.
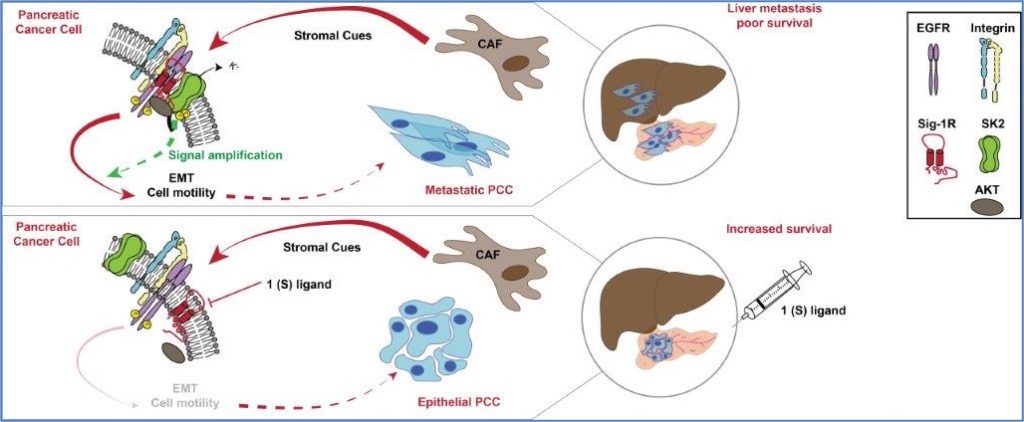
Sig-1R/KCNN2 channel complex sets a signalling hub bolstering CAF-triggered metastatic process in pancreatic cancer.Gut, 2022
Rona G, Zeke A, Miwatani-Minter B, de Vries M, Kaur R, Schinlever A, Garcia S.F, Goldberg H.V, Wang H, Hinds T.R, Bailly F, Zheng N, Cotelle P, Desmaële D, Landau N.R, Dittmann M, Pagano M.
The NSP14/NSP10 RNA repair complex as a Pan-coronavirus therapeutic target. Cell Death Differ 2022, 29, 285-292![]()
Sileo P, Simonin C, Melnyk P, Chartier-Harlin M-C, Cotelle P.
Crosstalk between Hippo pathway and Wnt pathway in Huntington’s disease and other neurodegenerative disorders. Cells 2022, 11(22), 3631![]()
Taymans J-M, Mutez E, Sibran W, Vandewynckel L, Deldycke C, Bleuse S, Marchand A, Sarchione A, Kreisler A, Simonin C, Koprich J, Baille G, Defebvre, Dujardin K, Destée A, Chartier-Harlin M-C.
Alterations in the LRRK2-Rab pathway in urinary extracellular vesicles as Parkinson's disease and pharmacodynamic biomarkers. NPJ Parkinson’s disease, 2022, in press
Thomas SE, McCarthy WJ, El Bakali J, Brown KP, Kim SY, Blaszczyk M, Mendes V, Abell C, Floto RA, Coyne AG, Blundell TL.
Structural Characterization of Mycobacterium abscessus Phosphopantetheine Adenylyl Transferase Ligand Interactions: Implications for Fragment-Based Drug Design. Front Mol Biosci 2022, 9, 880432![]()
Zagiel B, Melnyk P, Cotelle P.
Progress with YAP/TAZ-TEAD inhibitors: a patent review (2018-present). Expert Opin. Ther. Pat., 2022, 32 (8), 899-912. By invitation
Zhao F, Abdellaoui M, Hagui W, Ballarin M, Berthet J, Corcé V, Delbaere S, Dossmann H, Espagne A, Forte J, Jullien L, Le Saux T, Mouriès-Mansuy V, Ollivier C, Fensterbank L.
Reactant-Induced Photoactivation of In Situ Generated Organogold Intermediates Leading to Alkynylated Indoles via Csp2-Csp Cross-Coupling. Nat Commun 2022, 13, 2295![]()
2021
Cresto N, Gardier C, Gaillard MC, Gubinelli F, Roost P, Molina D, Josephine C, Dufour N, Auregan G, Guillermier M, Bernier S, Jan C, Gipchtein P, Hantraye P, Chartier-Harlin MC, Bonvento G, Van Camp N, Taymans JM, Cambon K, Liot G, Bemelmans AP, Brouillet E.
The C-Terminal Domain of LRRK2 with the G2019S Substitution Increases Mutant A53T α-Synuclein Toxicity in Dopaminergic, Neurons In Vivo. Int J Mol Sci, 2021 Jun 23, 22(13), 6760
Drouyer M, Bolliger MF, Lobbestael E, Van den Haute C, Emanuele M, Lefebvre R, Sibran W, De Wit T, Leghay C, Mutez E, Dzamko N, Halliday GM, Murayama S, Martoriati A, Cailliau K, Bodart JF, Chartier-Harlin MC, Baekelandt V, Nichols RJ,
Taymans J-M.
Protein phosphatase 2A holoenzymes regulate leucine-rich repeat kinase 2 phosphorylation and accumulation. Neurobiol Dis, 2021 Sep, 157, 105426
Ettaoussi M, Laversin A, Vreulz B, Rami M, Lebègue N, Delagrange P, Caignard D-H, Melnyk P, Liberelle M, Yous S.
Synthesis and SAR studies of Novel Isoquinoline and Tetrahydroisoquinoline Derivatives as Melatonin Receptor Ligands. Chem Med Chem, 2021, 17(3)
Fedorova OA, Arkhipova AN, Panchenko PA, Berthet J, Delbaere S, Minkovska, Fedorov Y-V.
Fluorescent photochromic complex of 1,8-naphthalimide derivative and benzopyrane containing benzo-18-crown-6 ether. J Photochem Photobiol A-Chem, 2021, 405
Gibault F, Bailly F, Sturbaut M, Coevoet M, Pugniez M, Magnez R, Thuru X, Melnyk P, Allemand F, Hong W, Pobbati A, Guichou JF, Cotelle P.
Design, Synthesis and Evaluation of a Series of 1,5-Diaryl-1,2,3-triazole-4-carbohydrazones as Inhibitors of the YAP-TAZ/TEAD Complex. Chem Med Chem, 2021, 16 (18), 2823-2844
Goveas L, Mutez E, Chartier-Harlin M-C, Taymans J-M.
Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells. Cells, 2021 Apr 22;10(5):981
Graca V, Berthet J, Sousa C-M, Delbaere S, Coelho P-J. Synthesis of Vinylnaphthofurans and NMR Analysis of their Photoswitching. Eur J Org Chem, 2021, 13, 1979-1988
Le Maréchal M, Mailles A, Seigneurin A, Tattevin P, Stahl JP, Épaulard O, Scientific Committee and Investigators Group.
A Prospective Cohort Study to Identify Clinical, Biological, and Imaging Features That Predict the Etiology of Acute Encephalitis. Clin Infect Dis 2021, 73(2), 264-270
Marc S, Mésangeau C, Coevoet M, Barczyk A, Burlet S, Verwaerde P, Brantis C, Sergeant N, Carato P, Melnyk P.
Pharmacomodulations around an anti-Alzheimer drug-candidate. Eur J Med Chem Rep, 2021, 04
Pacot L, Vidaud D, Sabbagh A, Laurendeau I, Briand-Suleau A, Coustier A, Maillard T, Barbance C, Morice-Picard F, Sigaudy S, Glazunova OO, Damaj L, Layet V, Quelin C, Gilbert-Dussardier B, Audic F, Dollfus H, Guerrot AM, Lespinasse J, Julia S, Vantyghem MC, Drouard M, Lackmy M, Leheup B, Alembik Y, Lemaire A, Nitschké P, Petit F, Dieux Coeslier A, Mutez E, Taieb A, Fradin M, Capri Y, Nasser H, Ruaud L, Dauriat B, Bourthoumieu S, Geneviève D, Audebert-Bellanger S, Nizon M, Stoeva R, Hickman G, Nicolas G, Mazereeuw-Hautier J, Jannic A, Ferkal S, Parfait B, Vidaud M, Members Of The Nf France Network, Wolkenstein P, Pasmant E.
Severe Phenotype in Patients with Large Deletions of NF1, Cancers (Basel). 2021, 13(12), 2963
Sarchione A, Marchand A, Taymans JM, Chartier-Harlin MC.
Alpha-Synuclein and Lipids: The Elephant in the Room?, Cells. 2021, 10(9), 2452
Shabajee-Alibay P, Bonnaud A, Malpaux B, Delagrange P, Audinot V, Yous S, Boutin JA, Stephan J-P, Leprince J, Legros C.
Putative new melatonin binding site in sheep brain, MTx: preliminary observations and characteristics
J Pharmacol Exp Therap, 2021, 380(1), 76-89
Spillier Q, Ravez S, Dochain S, Vertommen D, Thabault L, Feron O, Frédérick R.
Unravelling the Allosteric Targeting of PHGDH at the ACT-Binding Domain with a Photoactivatable Diazirine Probe and Mass Spectrometry Experiments. Molecules 2021, 26(2), 477
Stoup N, Liberelle M, Schulz C, Cavdarli S, Vasseur R, Magnez R, Lahdaoui F, Skrypek N, Peretti F, Frenois F, Thuru X, Melnyk P, Renault N, Jonckherre N, Lebegue N, Van Seuningen I.
The EGF domains of MUC4 oncomucin mediate HER2 binding affinity and promote pancreatic cancer cell tumorigenesis, Cancers, 2021, 13(22), 5746
Stoup N, Liberelle M, Schulz C, Vasseur R, Magnez R, Thuru X, Melnyk P, Renault N, Jonckherre N, Lebegue N, Van Seuningen I.
The EGF domains of MUC4 oncomucin interact with ErbB2 and mediate tumorigenic activity of cancer cells represent new potential therapeutic targets, The FASEB J. 2021; 35
Sturbaut M, Bailly F, Coevoet M, Sileo P, Pugniere M, Liberelle M, Magnez R, Thuru X, Chartier-Harlin MC, Melnyk P, Gelin M, Allemand F, Guichou JF, Cotelle P.
Discovery of a cryptic site at the interface 2 of TEAD – Towards a new family of YAP/TAZ-TEAD inhibitors, Eur J Med Chem, 2021, 226: 113835-849
Tautou M, Eddarkaoui S, Descamps F, Larchanché PE, El Bakali J, Goveas LM, Dumoulin M, Lamarre C, Blum D, Buée L, Melnyk P, Sergeant N.
A β-secretase modulator decreases Tau pathology and preserves short-term memory in a mouse model of neurofibrillary degeneration. Frontiers in Pharmacology, 2021,12, 679335![]()
Taymans JM,
Specialty Grand Challenge for Molecular Signalling and Pathways in Molecular Neuroscience. Front Mol Neurosci. 2021, 14, 694776
2020
Bachoud-Lévi. AC on behalf the Multicentric Intracerebral Grafting in Huntington's Disease Group.
Human Fetal Cell Therapy in Huntington's Disease: A Randomized, Multicenter, Phase II Trial.
Mov Disord. 2020, 35(8), 1323-1335
Belarbi K, Cuvelier E, Bonte MA, Desplanque M, Gressier B, Devos D, Chartier-Harlin MC.
Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol Neurodegener, 2020, 15, 59
Bolteau R, Descamps F, Ettaoussi M, Caignard D-H, Delagrange P, Melnyk P, Yous S.
Quinalozine and Phtalazine Derivatives as Novel Melantonin Receptor Ligands Analogues of Agomelatine. Eur J Med Chem, 2020, 189, 112078
Desplanque M, Bonte MA, Gressier B, Devos D, Chartier-Harlin MC, Belarbi K.
Trends in glucocerebrosides research: a systematic review. Front Physiology, 2020, 29, 11:558090
El-Kashef H, Badr G, Abo El-Maali N, Sayed D, Melnyk P, Lebègue N, Abd El-Khalek R.
Synthesis of a novel series of (Z)-3,5-disubstituted thiazolidine-2,4-diones as promising anti-breats cancer agents, Bioorg Chem, 2020, 96,103569
Hamdi I, Buntinx G, Aloïse S, Tiwari A K, Delbaere S, Takeshita M.
Cyclization Dynamics and competitive processes of photochromic perfluorocyclopentene dithienylethylene in solution, ChemPhysChem, 2020, 21, 2223-2229
Hurtevent A, Le Naour M, Leclerc V, Carato P, Melnyk P, Hennuyer N, Staels B, Beucher-Gaudin M, Dacquet C, Lebegue N.
6-Benzoyl-benzothiazol-2-one as an important scaffold on the pharmacological profile of α-alkoxyphenylpropionic acid derived PPAR agonists, J Enzyme Inhib Med Chem, 2020, 35(1), 524-538
Keo A, Mahfouz A, Ingrassia AMT, Meneboo JP, Villenet C, Mutez E, Comptdaer T, Lelieveldt BPF, Figeac M, Chartier-Harlin MC, van de Berg WDJ, van Hilten JJ, Reinders MJT.
Transcriptomic signatures of brain regional vulnerability to Parkinson's disease, Commun Biol, 2020, 3(1),101
Kreisler A, Simonin C, Degardin A, Mutez E, Defebvre L.
Anatomy-guided injections of botulinum neurotoxin in neck muscles: how accurate is needle placement? Eur J Neurol, 2020, 27(11), 2142-46
Leguen C, Mazzah A, Penhoat M, Melnyk P, Rolando C, Chausset-Boissarie L.
Direct and regioselective fluorination of pyridylic and quinolinic C(sp3)-H bonds with selectfluor, Synthesis, 2020, 53 (06), 1157-1162
Liberelle M, Jonckheere N, Melnyk P, Van Seuningen I, Lebegue N.
EGF-containing membrane-bound Mucins : a hidden ErbB2 signaling pathway ? J Med Chem, 2020, 63(10), 5074-5088
Lobbestael E, Van den Haute C, Macchi F, Taymans JM, Baekelandt V.
Pathogenic LRRK2 requires secondary factors to induce cellular toxicity, Bioscience Rep, 2020, 40(10), BSR20202225
Mangone G, Bekadar S, Cormier-Dequaire F, Tahiri K, Welaratne A, Czernecki V, Pineau F, Karachi C, Castrioto A, Durif F, Tranchant C, Devos D, Thobois S, Meissner WG, Navarro MS, Cornu P, Lesage S, Brice A, Welter ML, Corvol JC.
Early cognitive decline after bilateral subthalamic deep brain stimulation in Parkinson's disease patients with GBA mutations.
Parkinsonism Relat Disord. 2020, 76, 56-62
Marchand A, Drouyer M, Sarchione A, Chartier-Harlin MC, Taymans JM.
LRRK2 Phosphorylation, More Than an Epiphenomenon, Front Neurosci, 2020, 14,527
Norel L, Bernot K, Gendron F, Gould C, Roisnel T, Delbaere S, Le Guennic B, Jacquemin D, Rigaut S.
Coordination-enhanced photochromism in dysprosium dinuclear complexes with photomodulated single-molecule magnet behavior, Chem2, 2020, 4, 2
Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Fabbri M, Meissmer WG, Rachdi A, Tison F, Perez-Lloret S for the COPARK study group.
Excessive buccal saliva in patients with Parkinson''s disease of the French COPARK cohort, J Neural Transm (Vienna) 2020, 127, 1607-1617
Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Fabbri M, Meissmer WG, Rachdi A, Tison F, Perez-Lloret S for the COPARK study group.
Utilization Patterns of Amantadine in Parkinson''s Disease Patients Enrolled in the French COPARK Study, Drugs Aging 2020, 37, 215-223
Ribeiro JA, Hammer A, Libreros-Zúñiga GA, Chavez-Pacheco SM, Tyrakis P, de Oliveira GS, Kirkman T, El Bakali J, Rocco SA, Sforça ML, Parise-Filho R, Coyne AG, Blundell TL, Abell C, Dias MVB.
Using a fragment-based approach to identify alternative chemical scaffolds targeting dihydrofolate reductase from Mycobacterium tuberculosis, ACS Infect Dis, 2020, 6(8), 2192-2201
Rideout HJ, Chartier-Harlin MC, Fell MJ, Hirst WD, Huntwork-Rodriguez S, Leyns CEG, Mabrouk OS, Taymans JM.
The Current State-of-the Art of LRRK2-Based Biomarker Assay Development in Parkinson's Disease, Front Neurosci, 2020, 14, 865
Spillier Q, Ravez S, Unterlass J, Corbet C, Degavre C, Feron O, Frédérick R.
Structure-Activity Relationships (SARs) of α-Ketothioamides as Inhibitors of Phosphoglycerate Dehydrogenase (PHGDH), Pharmaceuticals (Basel), 2020, 13(2), 20
2019
Aibidi Y, Guerrin C, Aleveque O, Leriche P, Delbaere S, Sanguinet L, Champagne B.
BT-2-BOX: an assembly toward multi-modal and multi-level molecular system simple as a breeze, J Phys Chem C, 2019, 123, 11823-11832
Berthet J, Agouridas L, Chen S, Allouchi H, Melnyk P, Champagne B, Delbaere S.
Synthesis and Photoswitching properties of new derivatives of azoresveratrol, Dyes Pigment, 2019, 171, 107666
Blaise AS, Baille G, Carrière N, Devos D, Dujardin K, Grolez G, Kreisler A, Kyheng M, Moreau C, Mutez E, Seguy D, Lefebvre L.
Safety and effectiveness of levodopa-carbidopa intestinal gel for advanced Parkinson's disease: A large single-center study, Rev Neurol (Paris) 2020, 176 (4), 268-276
Braisch U, Muche R, Rothenbacher D, Landwehrmeyer GB, Long JD, Orth M, REGISTRY Investigators of the European Huntington's Disease Network and COHORT Investigators of the Huntington Study Group.
Identification of symbol digit modality test score extremes in Huntington's disease, Am J Med Genet B Neuropsychiatr Genet 2019, 180(3), 232-245
Colosimo C, Charles D, Misra VP, Maisonobe P, Om S, on behalf of the INTEREST IN CD2 study group.
How satisfied are cervical dystonia patients after 3 years of botulinum toxin type A treatment? Results from a prospective, long-term observational study, J Neurol 2019, 266, 3038-3046
Fernández B, Lara Ordóñez AJ, Fdez E, Mutez E, Comptdaer T, Leghay C, Kreisler A, Simonin C, Vandewynckel L, Defebvre L, Destée A, Bleuse S, Taymans JM, Chartier-Harlin MC, Hilfiker S.
Centrosomal cohesion deficits as cellular biomarker in lymphoblastoid cell lines from LRRK2 Parkinson's disease patients, Biochemical J, 2019, 476(19), 2797-2813
Graça VC, Calhelha RC, Nunes FM, Berthet J, Ferreira ICFR, Santos PF.
Isolation of secondary metabolites from Geranium molle L. with anticancer potential, Ind Crops Prod, 2019, 142, 1-5
Guerrin C, Aibidi Y, Sanguinet L, Leriche P, Aloise S, Orio M, Delbaere S.
When light and acid play Tic-Tac-Toe with a nine-state molecular switch, J Am Chem Soc, 2019, 141, 19151-19160
Hamdi I, Buntinx G, Poizat O, Delbaere S, Perrier A, Rikiya Y, Ken-ichi M, Takeshita M, Aloïse S.
Unraveling Ultrafast Dynamics of the Photoswitchable Bridged Dithienylethenes Under Structural Constraints, Phys Chem Chem Phys, 2019, 21, 6407-6414
Issa S, Prandina A, Bedel N, Rongved P, Yous S, Le Borgne M, Bouaziz Z.
Carbazole scaffolds in cancer therapy: a review from 2012 to 2018, J Enzyme Inhib Med Chem, 2019, 34(1), 1321-1346
Johansson LC, Stauch B, McCorvy JD, Han GW, Patel N, Huang XP, Batyuk A, Gati C, Slocum ST, Li C, Grandner JM, Hao S, Olsen RHJ, Tribo AR, Zaare S, Zhu L, Zatsepin NA, Weierstall U, Yous S, Stevens RC, Liu W, Roth BL, Katritch V, Cherezov V.
XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity, Nature, 2019, 569(7755), 289-292
Liberelle M, Magnez R, Thuru X, Bencheikh Y, Ravez S, Quenon C, Drucbert AS, Foulon C, Melnyk P, Van Seuningen I, Lebegue N.
MUC4-ErbB2 Oncogenic Complex: Binding studies using Microscale Thermophoresis and Surface Plasmon Resonance, Sci Rep, 2019, 9, 16678-85
Rami M, Winum JY, Supuran C, Melnyk P, Yous S.
(Hetero)aryl substituted thaizol-2,4-yl scaffold as human carbonic anhydrase I, II, VII and XIV activators, J Enzyme Inhib Med Chem, 2019, 34 (1), 224-229
Saez-Ayala M, Laban Yekwa E, Mondielli C, Roux L, Hernández S, Bailly F, Cotelle P, Rogolino D, Canard B, Ferron F, Alvarez K.
Metal chelators for the inhibition of the lymphocytic choriomeningitis virus endonuclease domain, Antivir Res 2019, 162, 79-89
Sergeant N, Vingtdeux V, Eddarkaoui S, Gay M, Evrard C, Lefur N, Laurent C, Caillierez R, Obriot H, Larchanché PE, Farce A, Coevoet M, Carato P, Kouach M, Descat A, Dallemagne P, Buée-Scherrer V, Blum D, Hamdane M, Buée L, Melnyk P.
New piperazine multi-action drugs prevent neurofibrillary degeneration and amyloid deposition, and preserve memory in animal models of Alzheimer’s disease, Neurobiol Dis 2019, 129, 217-233
Sousa CM, Berthet J, Delbaere S, Coelho PJ.
Enhancement of the color intensity of photochromic fused-naphthopyrans, Dyes Pigment 2019, 169, 118-124
Spillier Q, Vertommen D, Ravez S, Marteau R, Thémans Q, Corbet C, Feron O, Wouters J, Frédérick R.
Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation, Sci Rep 2019, 9(1), 4737
Stauch B, Johansson LC, McCorvy JD, Patel N, Han GW, Huang XP, Gati C, Batyuk A, Slocum ST, Ishchenko A, Brehm W, White TA, Michaelian N, Madsen C, Zhu L, Grant TD, Grandner JM, Shiriaeva A, Olsen RHJ, Tribo AR, Yous S, Stevens RC, Weierstall U, Katritch V, Roth BL, Liu W, Cherezov V.
Structural basis of ligand recognition at the human MT1 melatonin receptor, Nature 2019, 569(7755), 284-288
Saez-Ayala M, Laban Yekwa E, Mondielli C, Roux L, Hernández S, Bailly F, Cotelle P, Rogolino D, Canard B, Ferron F, Alvarez K.
Metal chelators for the inhibition of the lymphocytic choriomeningitis virus endonuclease domain. Antivir. Res., 2019, 162, 79-89
Sergeant N, Vingtdeux V, Eddarkaoui S, Gay M, Evrard C, Lefur N, Laurent C, Caillierez R, Obriot H, Larchanché PE, Farce A, Coevoet M, Carato P, Kouach M, Descat A, Dallemagne P, Buée-Scherrer V, Blum D, Hamdane M, Buée L, Melnyk P.
New piperazine multi-action drugs prevent neurofibrillary degeneration and amyloid deposition, and preserve memory in animal models of Alzheimer’s disease. Neurobiol Disease, 2019, S0969-9961, 30327-9
Sousa CM, Berthet J, Delbaere S, Coelho PJ.
Enhancement of the color intensity of photochromic fused-naphthopyrans, Dyes Pigment, 2019, 169, 118-124
Spillier Q, Vertommen D, Ravez S, Marteau R, Thémans Q, Corbet C, Feron O, Wouters J, Frédérick R.
Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation, Sci Rep, 2019, 9(1), 4737
Stauch B, Johansson LC, McCorvy JD, Patel N, Han GW, Huang XP, Gati C, Batyuk A, Slocum ST, Ishchenko A, Brehm W, White TA, Michaelian N, Madsen C, Zhu L, Grant TD, Grandner JM, Shiriaeva A, Olsen RHJ, Tribo AR, Yous S, Stevens RC, Weierstall U, Katritch V, Roth BL, Liu W, Cherezov V.
Structural basis of ligand recognition at the human MT1 melatonin receptor, Nature, 2019, 569(7755), 284-288
2018
Boot E, Butcher NJ, Udow S, Marras C, Mok KY, …., Mutez E, …, Bassett AS, International Research Group on 22q11.2DS-associated Parkinson's Disease. Typical features of Parkinson disease and diagnostic challenges with microdeletion 22q11.2, Neurology, 2018, 90(23), e2059-e2067.
Boulahjar R, Rincon Arias A, Bolteau R, Renault N, Coevoet M, Barczyk A, Duroux R, Yous S, Melnyk P, Agouridas L, Design and synthesis of 2,6-disubstituted-8-amino imidazo[1,2a]pyridines, a promising privileged structure, Bioorg Med Chem, 2018, 26, 3296-3307.
Cuvelier E, Méquinion M, Leghay C, Sibran W, Stievenard A, Sarchione A, Bonte MA, Vanbesien-Mailliot C, Viltart O, Saitoski K, Caron E, Labarthe A, Comptdaer T, Semaille P, Carrié H, Mutez E, Gressier B, Destée A, Chartier-Harlin MC*, Belarbi K*, Overexpression of Wild-Type Human Alpha-Synuclein Causes Metabolism Abnormalities in Thy1-aSYN Transgenic Mice, Front Mol Neurosci, 2018, 11, 321
Duroux R, Agouridas L, Renault N, El Bakali J, Furman C, Melnyk P, Yous S, Antagonists of the aden osine A(2A) receptor based on a 2-arylbenzoxazole scaffold: Investigation of the C5-and C7-positions to enhance affinity, Eur J Med Chem, 2018, 144, 151-163
Evrard C, Kieulen-Campard P, Coevoet M, Opsomer R, Tasiaux B, Melnyk P, Octave JN, Buée L, Sergeant N, Vingtdeux V, Contribution of the endosomal-lysosomal and proteosomal systems in amyloid-β precursor protein derived fragments processing, Front Cell Neurosci, 2018, 12, 435
Gay M, Carato P, Coevoet M, Renault N, Larchanché PE, Barczyk A, Yous S, Buée L, Sergeant N, Melnyk P, New phenylaniline derivatives as modulators of amyloid protein precursor metabolism, Bioorg Med Chem, 2018, 26, 2151-64
Gay M, Evrard C, Descamps F, Carato P, Renault N, Coevoet M, Eddarkaoui S, Baud C, Larchanché PE, Buée L, El Bakali J, Vingtdeux V, Sergeant N, Melnyk P, A Phenotypic Approach to the Discovery of Compounds that Promote Non-Amyloidogenic Processing of the Amyloid Precursor Protein: Toward a New Profile of Indirect β-Secretase Inhibitors, Eur J Med Chem, 2018, 159, 104-125
Gibault F, Sturbaut M, Bailly F, Melnyk P, Cotelle P, Targeting Transcriptional Enhanced Associate Domains (TEADs), J Med Chem, 2018, 61(12), 5057-5072
Gibault F, Coevoet M, Sturbaut M, Farce A, Renault N, Allemand F, Guichou JF, Drucbert AS, Foulon C, Magnez R, Thuru X, Corvaisier M, Huet G, Chavatte P, Melnyk P, Bailly F, Cotelle , Towards the Discovery of a Novel Class of YAP-TEAD Interaction Inhibitors by Virtual Screening Approach Targeting YAP-TEAD Protein-Protein Interface, Cancers, 2018, 10(5), 140 Invitation paper.
Guerrin C, Szalóki G, Berthet J, Sanguinet L, Orio M, Delbaere S, Indolino-oxazolidine Acido and Photochromic System investigated by NMR and DFT Calculations, J Org Chem, 2018, 82, 12028-12037
Hamdi I, Buntinx G, Poizat O, Perrier A, Le Bras L, Delbaere S, Barrau S, Louati M, Takeshita M, Tokushige K, Takao M, Aloïse S, Excited-State Dynamics of Dithienylethenes Functionalized for Self-Supramolecular Assembly, J Phys Chem A, 2018, 122, 3572-3582
Madero-Pérez J, Fdez E, Fernández B, Lara Ordóñez AJ, Blanca Ramírez M, Gómez-Suaga P, Waschbüsch D, Lobbestael E, Baekelandt V, Nairn AC, Ruiz-Martínez J, Aiastui A, López de Munain A, Lis P, Comptdaer T, Taymans JM, Chartier-Harlin MC, Beilina A, Gonnelli A, Cookson MR, Greggio E, Hilfiker S, Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation, Mol Neurodegener, 2018, 13, 3
Mohamed SH, Quertinmont J, Delbaere S, Sanguinet L, Champagne B, Assessing the Structure of Octastate Molecular Switches Using H-1 NMR Density Functional Theory Calculations, J Phys Chem C, 2018, 122, 1800-1808
Sousa CM, Berthet J, Delbaere S, Coelho PJ, Synthesis of Polycyclic Spironaphthofuran Derivatives by Acid-Catalyzed Domino Reaction of 2-Naphthols with Tetraarylbut-2-yne-1,4-diols, Eur J Org Chem, 2018, 3291-97
Taymans JM, Chartier-Harlin MC, In silico and Wet Bench Interactomics Sheds Light on the Similitudes and Differences between Human ROCO Proteins, Proteomics, 2018, e1800103
2017
Belarbi K, Cuvelier E, Destee A, Gressier B, Chartier-Harlin MC, NADPH oxidases in Parkinson's disease: a systematic review, Mol. Neurodegener, 2017, 12, 84
Berdnikova DV, Aliyeu TM, Delbaere S, Fedorov YV, Jonusauskas G, Novikov VV, Pavlov AA, Peregudov AS, Shepel NE, Zubkov FI, Fedorova OA, Regio- and stereoselective [2+2] photocycloaddition in Ba2+ templated supramolecular dimers of styryl-derivatized aza-heterocycles, Dyes Pigment, 2017, 139, 397-402
Civiero L, Cogo S, Kiekensl A, Morganti C, Tessari I, Lobbestael E, Baekelandt V, Taymans JM, Chartier-Harlin MC, Franchin C, Arrigoni G, Lewis PA, Piccoli G, Bubacco L, Cookson MR, Pinton P, Greggio E, PAK6 Phosphorylates 14-3-3 gamma to Regulate Steady State Phosphorylation of LRRK2, Front Molec Neurosci, 2017, 10, 417
Donnier-Marechal M, Carato P, Larchanche PE, Ravez S, Boulahjar R, Barczyk A, Oxombre B, Vermersch P, Melnyk P , Synthesis and pharmacological evaluation of benzamide derivatives as potent and selective sigma-1 protein ligands, Eur J Med Chem, 2017, 138, 964-978
Duroux R, Rami M, Landagaray E, Ettaoussi M, Caignard DH, Delagrange, Melnyk P, Yous S, Synthesis and biological evaluation of new naphtho- and quinolinocyclopentane derivatives as potent melatoninergic (MT1/MT2) and serotoninergic (5-HT2C) dual ligands, Eur J Med Chem, 2017, 141, 552-566
Duroux R, Renault N, Cuelho JE, Agouridas L, Blum D, Lopes LV, Melnyk P, Yous S, Design, synthesis and evaluation of 2-aryl benzoxazoles as promising hit for the A2A receptor, J Enzyme Inhib Med Chem, 2017, 32, 850-864
Duroux R, Ciancetta A, Mannes P, Yu JH, Boyapati S, Gizewski E, Yous S, Ciruela F, Auchampach JA, Gao ZG, Jacobson KA, Bitopic fluorescent antagonists of the A(2A) adenosine receptor based on pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine functionalized congeners, MedChemComm, 2017, 8, 1659-1667
Edwards TC, Lomonosova E, Patel JA, Li Q, Villa JA, Gupta AK, Morrison LA, Bailly F, Cotelle P, Giannakopoulou E, Zoidis G, Tavis JE , Inhibition of hepatitis B virus replication by N-hydroxyisoquinolinediones and related polyoxygenated heterocycles, Antiviral Res, 2017, 143, 205-217
Gibault F, Bailly F, Corvaisier M, Coevoet M, Huet G, Melnyk P, Cotelle P, Molecular Features of the YAP Inhibitor Verteporfin: Synthesis of Hexasubstituted Dipyrrins as Potential Inhibitors of YAP/TAZ, the Downstream Effectors of the Hippo Pathway, ChemMedChem, 2017, 12, 954-961
Guérin J, Léaustic A, Berthet J, Métivier R, Guillot R, Delbaere S, Nakatani K, Yu P, Light-Controlled Release and Uptake of Zinc Ions in Solution by a Photochromic Terthiazole-Based Ligand, Chem Asian J, 2017, 12, 853-859
Hensman Moss DJ, Pardiñas AF, Langbehn D, Lo K, Leavitt BR, Roos R, Durr A, Mead S, … Destee A, …..Simonin C, … Tabrizi SJ, Identification of genetic variants associated with Huntington's disease progression: a genome-wide association study, Lancet Neurol, 2017, 16, 701-711
Landagaray E, Ettaoussi M, Rami M, Boutin JA, Caignard DH, Delagrange P, Melnyk P, Berthelot P, Yous S, New quinolinic derivatives as melatonergic ligands: Synthesis and pharmacological evaluation, Eur J Med Chem, 2017, 127, 621-631
Méquinion M, Le Thuc O, Zgheib S, Alexandre D, Chartrel N, Rovère C, Hardouin P, Viltart O, Chauveau C, Long-Term Energy Deficit in Mice Causes Long-Lasting Hypothalamic Alterations after Recovery, Neuroendocrinology, 2017,105(4), 372-383
Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, , Therapeutic potential of autophagy-enhancing agents in Parkinson's disease, Mol Neurodegener, 2017,12 (1):11
Nkiliza A, Chartier-Harlin MC, ATXN2 a culprit with multiple facets, Oncotarget, 2017, 8, 34028-29
Pirat C, Dacquet C, Leclerc V, Hennuyer N, Beucher-Gaudin M, Zanirato G, Géant A, Staels B, Ktorza A, Farce A, Caignard DH, Berthelot P, Lebègue N, Anti-diabetic activity of fused PPAR gamma-SIRT1 ligands with limited body-weight gain by mimicking calorie restriction and decreasing SGK1 expression, Eur J Med Chem, 2017, 137, 310-326
Ramirez MB, Lara Ordonez AJ, Fdez E, Madero-Perez J, Gonnelli A, Drouyer M, Chartier-Harlin MC, Taymans JM, Bubacco L, Greggio E, Hilfiker S, GTP binding regulates cellular localization of Parkinson's disease-associated LRRK2, Hum Mol Genet, 2017, 26, 2747-2767
Rassu M, Del Giudice MG, Sanna S, Taymans JM, Morari M, Brugnoli A, Frassineti M, Masala A, Esposito S, Galioto M, Valle C, Carri MT, Biosa A, Greggio E, Crosio C, Iaccarino C, Role of LRRK2 in the regulation of dopamine receptor trafficking, PLoS One, 2017, 12, e0179082
Ravez S, Corbet C, Spillier Q, Dutu AD, Mullarky E, Cantley LC, Feron O, Frédérick R, alpha-Ketothioamide Derivatives: A Promising Tool to Interrogate Phosphoglycerate Dehydrogenase (PHGDH), J Med Chem, 2017, 1591-1597
Ravez S, Spillier Q, Marteau R, Feron O, Frédérick R, Challenges and Opportunities in the Development of Serine Synthetic Pathway Inhibitors for Cancer Therapy, J Med Chem, 2017, 1227-1237
Sanguinet L, Berthet J, Szaloki G, Aleveque O, Pozzo JL, Delbaere S, 13 metastable states arising from a simple multifunctional unimolecular system, Dyes Pigment, 2017, 137, 490-498
Sejwal K, Chami M, Rémigy H, Vancraenenbroeck R, Sibran W, Sütterlin R, Baumgartner P, McLeod R, Chartier-Harlin MC, Baekelandt V, Stahlberg H, Taymans JM, Cryo-EM analysis of homodimeric full-length LRRK2 and LRRK1 protein complexes, Sci Rep, 2017, 7, 8667
Sousa CM, Berthet J, Delbaere S, Polonia A, Coelho PJ, Control of the Switching Speed of Photochromic Naphthopyrans through Restriction of Double Bond Isomerization, J Org Chem, 2017, 82, 12028-12037
Sousa CM, Berthet J, Delbaere S, Coelho PJ, A closer look at the photochromism of vinylidene-naphthofurans, Dyes Pigment, 2017, 137, 593-600
Stievenard A, Méquinion M, Andrews ZB, Destée A, Chartier-Harlin MC, Viltart O, Vanbesien-Maillot CC, Is there a role for ghrelin in central dopaminergic systems? Focus on nigrostriatal and mesocorticolimbic pathways, Neurosci Biobehav Rev, 2017, 73: 255-275
Taymans JM.
Regulation of LRRK2 by Phosphatases, Adv Neurobiol, 2017, 14, 145-160
Taymans JM, Mutez E, Drouyer M, Sibran W, Chartier-Harlin MC.
LRRK2 detection in human biofluids: potential use as a Parkinson's disease biomarker?, Biochem Soc Trans, 2017, 45, 207-212
Verny C, Bachoud-Lévi AC, Durr A, Goizet C, Azulay JP, Simonin C, Tranchant C, Calvas F, Krystkowiak P, Charles P, Youssov K, Scherer C, Prundean A, Olivier A, Reynier P, Saudou F, Maison P, Allain P, von Studnitz E, Bonneau D; CYST-HD Study Group, A Randomized, Double-Blind, Placebo-Controlled Trial Evaluating Cysteamine in Huntington's Disease, Mov Disord, 2017, 32, 932-936
Pattents
Pattents deposited
Allemand F, Cotelle P, Gelin M, Guichou JF, Lebègue N, Melnyk P, Toulotte F, Zagiel B, Arylalkyloxyindole compounds and derivatives and their use, EP 22305235.8 - 1st of March 2022.
Taymans J-M, Mutez E, Chartier-Harlin M-C, Early and non-invasive method for assessing a subject’s risk of having Parkinson’s disease, EP22305293.7 – 15th of March 2022.
Borgese F, Melnyk P, Rapetti-Mauss R, Soriani O, Tomasini R, Sigma-1 ligands for the treatment of cancer, EP22306212.6 – 10Th of August 2022.
Mésangeau C, Carato P, Renault N, Sergeant N, Melnyk P, Diphenylpyrazole compounds and their use, EP22306550.9 – 12th of October 2022
- Melnyk P, Carato P, Burlet S, Nguyen TH, Verwaerde P, Sergeant N, Estrella C. γ-carbolines derivatives for the treatment of neurodegenerative diseases, EP 13305927.9
- Melnyk P, Carato P, Burlet S, Nguyen TH, Verwaerde P, Sergeant N, Estrella C. β-carbolines derivatives for the treatment of neurodegenerative diseases, EP 13305928.7
- Carato P, Descamps F, El Bakali J, Evrard C, Gay M, Melnyk P, Renault N, Sergeant N, Vingtdeux V. Polyamino biaryl compounds and their use. EP 18305932.8
Accepted Pattents
- Melnyk P, Carato P, Burlet S, Nguyen TH, Verwaerde P, Sergeant N, Estrella C. Beta and gamma-carbolines derivatives for the treatment of neurodegenerative diseases, PCT/EP2014/063771. WO2014/207240. PCT/EP2015/063370
- Melnyk P, Carato P, Burlet S, Nguyen TH, Verwaerde P, Sergeant N, Estrella C. Na-substituted carbolines derivatives for the treatment of neurodegenerative diseases, EP 13305929.5, PCT/EP2014/063772, WO2014/207241
- Melnyk P, Vermersch P, Carato P, Vanteghem-Oxombre B, Zephir H, Donnier-Maréchal M. Compounds, pharmaceutical composition and their use in treating neurodegenerative diseases, EP14305919.4, PCT/EP2015/063370. Licence en discussion
- Tavis JE, Cotelle P, Bailly F. N-Hydroxyisoquinolindione inhibitors of HBV replication. PCT/US2017/022738
Licensed Pattents
- Melnyk P, Sergeant N, Buée L, Delacourte A. Use of 1,4-bis(3-aminoalkylyl)piperazine derivatives in the treatment of neurodegenerative diseases, WO 2006 051489, PCT/IB2005/053676
Licence exclusive accordée à AlzProtect dans le domaine des maladies neurodégénératives.
Molécule actuellement en phase 2 chez l’homme. - Barrier M, Buée L, Burlet S, Delacourte A, Estrella C, Melnyk P, Sergeant N, Verwaerde P. Sulfate salts of N-(3-(4-(3-(diisobutylamino)propyl)piperazin-1-yl)propyl)-1H-benzo[d]imidazol-2-amine, preparation thereof and use of the same. WO2014/102339.
Licence exclusive accordée à AlzProtect dans le domaine des maladies neurodégénératives.
Molécule actuellement en phase 2 chez l’homme. - Melnyk P, Le Fur N, Gay M, Sergeant N, Buée L. Novel 1,4-bis(3-aminopropyl)piperazine derivative and its use . EP 15305384.8. PCT/EP2016/055633. Licence exclusive accordée à AlzProtect dans le domaine des maladies neurodégénératives.
PhD thesis
PhD in progress
- Alessia Sarchione (4th year) under the supervision of Dr MC Chartier-Harlin
"Defects in membrane trafficking in Parkinson's disease" - Andriana FIGUEROA-GARCIA (3rd year) under the supervision of Dr MC Chartier-Harlin
"Targeting translation during alpha-synuclein overexpression in Parkinson's disease" - Liesel Mary GOVEAS (3rd year) under the supervision of Dr JM Taymans
"Targeting of the kinase of Parkinson's disease “Leucine Rich repeat kinase 2” (LRRK2)" - Pasquale SILEO (3rd year) under the supervision of Pr P Cotelle
"Study of the Hippo pathway in Huntington's disease" - Nicolas STOUP (3rd year) under the co-supervision of Dr I Van Seuningen / Pr N Lebègue
" The MUC4-ErbB2 complex: from the structure-function relationship towards therapeutic targeting" - Alexandre GOBERT (2nd year) under the supervision of Pr N Lebègue
" Design, synthesis and evaluation of new activators of SIRT1 in neurodegenerative diseases" - Amélie LARVIN (1st year) under the supervision of Dr S Yous
"Design, synthesis and evaluation of potential A2A receptor antagonists and dual ligands targeting A2A / mGlu5 receptors in the treatment of neurodegenerative diseases" - Justine PETERS (1st year) under the supervision of Pr N Lebègue
"Design, synthesis and pharmacological evaluation of new antimalarial compounds"
Defended PhD since 2017
- Florine TOULOTTE (defended the 17/10/2021) under the supervision of Pr P Cotelle
"Synthesis and design of selective modulators of the YAP (TAZ) -TEAD interaction" - Antoine MARCHAND (defended the 09/10/2021) under the supervision of Dr JM Taymans
"LRRK2 and vesicular trafficking deficits in Parkinson's disease" - Marie TAUTOU (defended the 29/10/2021)
« Mise en évidence du mode d'action de molécules anti-Alzheimer et recherche de leur cible ». Co-supervision of Dr N Sergeant / Pr P Melnyk - Raphael BOLTEAU (defended the 02/10/2020)
« Conception, synthèse et évaluation pharmacologique d’antagonistes des récepteurs A2A et de ligands duaux ciblant les récepteurs A2A et mGlu5». Supervision of Dr S Yous - Maxime LIBERELLE (defended the 10/10/2019)
« Focus on the protein-protein interface of MUC4 / ErbB2 complex : a structural approach». Supervision of Pr N Lebègue - Florian DESCAMPS (defended the 03/10/2019)
« Conception, Synthèse et Evaluation de molécules interagissant avec la protéine VCP pour le traitement de maladies neurodégénératives». Supervision of Pr P Melnyk - Manon STURBAUT (defended the 27/09/2019)
« Conception, Synthèse et Evaluation d’inhibiteurs du complexe protéique YAP-TEAD». Supervision of Pr P Cotelle - Mathieu DROUYER (defended the 20/12/2018)
« Ciblage de la kinase parkinsonnienne LRRK2». Supervision of Dr JM Taymans - Clément GUERRIN (defended the 28/09/2018)
« Etude des propriétés de photocommutation de composés photoactivables couplée à une irradiation in situ et par calculs TD-DFT». Supervision of Pr S Delbaere (LASIR) - Floriane GIBAULT (defended the 13/10/2017)
« Conception, Synthèse et Evaluation d’inhibiteurs du complexe protéique YAP-TEAD». Supervision of Pr P Cotelle - Romain DUROUX (defended the 22/09/2017)
« Conception, Synthèse et Evaluation d’antagonistes des récepteurs A2A». Supervision of Dr S Yous - Elodie CUVELIER (defended in 2017)
« Modulation pharmacologique de la glucocérébrosidase chez un modèle murin de syndrome parkinsonien surexprimant l’alpha-synucléine». Supervision of Dr K Belarbi