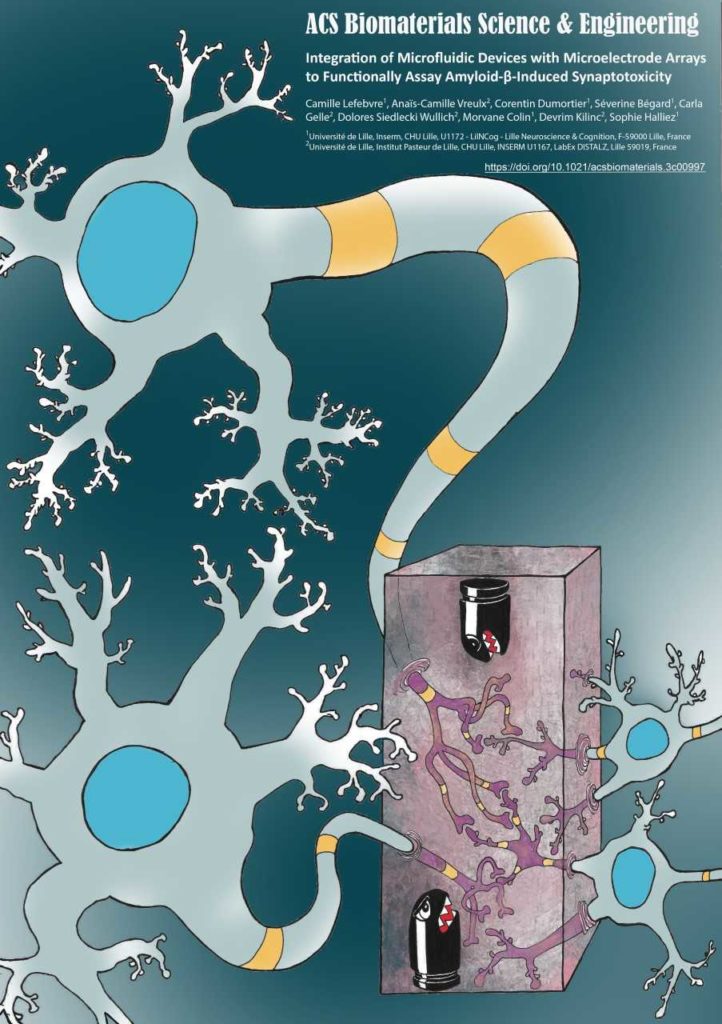
Alzheimer
and Tauopathies

Presentation
“Alzheimer & Tauopathies” Tean belongs to the “Lille Neuroscience & Cognition” and is located Lille, France.
Our team is interested in pathophysiological mechanisms leading to dementia and is composed of 16 permanent researchers: full time research scientists, professors and associate professors and clinicians. We also welcome postdoctoral fellows and MSc/PhD students. Our work focuses on Tau, protein involved in the physiopathology of Alzheimer’s disease and related disorders referred to as Tauopathies, going from the gene to the clinic and from basic research to biomedical application.
“Alzheimer & Tauopathies” team is interested in molecular and pathophysiological mechanisms of Tau pathophysiology. Our main contribution has been on the characterization of neurodegenerative disorders with dysfunction in Tau metabolism (expression, splicing, etc.) and the development of experimental models. We also demonstrated that Tau proteins are biomarkers of these disorders and correlated to neuronal death.
Our current research should lead to new diagnostic and therapeutic strategies of Alzheimer’s disease and related disorders. To the level of the etiopathogenesis of Alzheimer’s disease, the understanding of the relationships between amyloid pathology and Tau pathology is our main goal. Any molecular actors potentially involved in one of these pathways are evaluated in our experimental “humanized” models, as well as the related pharmacological aspects.
NEWS
David Blum winner of the Rachel Ajzen and Léon Iagolnitzer Prize 2023
Hope of treatment

Thierry Lhermitte for CNews: “Luc Buée’s trial concerns a currently unknown form of the Tau protein”… Read the article

Immunotherapies, a concrete turning point in research into Alzheimer’s disease.
“What we are experiencing today is the realization of a therapeutic hypothesis.”. Luc Buée
World Alzheimer's Day

Participation of David Blum and Luc Buée in the EMBO Workshop “Neural development and neurodegeneration”


Academia Sinica, Taipei, 2-4 décembre 2022
Marine Denéchaud
Award of the prestigious i-PhD innovation competition
Drinking two to three cups of coffee a day would have virtues for the memory and the heart.
Coffee drinkers may be at lower risk of premature death

Steinert’s disease is caused by the abnormal repetitions of a small DNA sequence in the DMPK gene.
© Unsplash
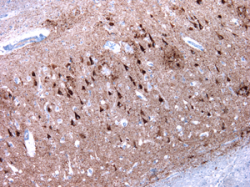
Anti-pTau labeling of neurofibrillary degeneration in the hippocampus in a patient with Alzheimer’s disease.
VIDEO
Alzheimer’s disease, the view of expert Florence Pasquier
They are the inserm
Alzheimer’s disease: the challenges of research deciphered by 3 researchers supported by the FRM
- STAFF
- RESEARCH INTEREST
- PUBLICATIONS
Researchers
 | DIRECTOR |
 | David Blum. |
 | Marie-Christine Galas, PhD. |
 | Nicolas Sergeant, PhD. Inserm research professor. University of Lille, Faculty of Medicine Research : Pathophysiology of Tauopathies, biomarkers & new therapeutic targets. |
Associate Professors/Professors – University-Associated | |
 | Dr. Valérie Buée-Scherrer, PhD. Associate Professor (HDR-Docent) University Artois, School of Sciences, Lens, France. Teaching: Cell Biology. Research : Molecular characterization of brain lesions found in neurodegenerat disorders. Studies in human and aivenimal models. Methods: Biochemistry, Cell biology, Molecular Biology, Immunohistochemistry. |
 | Dr. Morvane Colin, PhD. Associate Professor (HDR-Docent) University of Lille, Faculty of Medicine. Teaching: Cell Biology, Gene therapy & vectorology. Research: “Tau spreading” Cell to cell transfer process; immunotherapy anti-tau, neuronal network. Dedicated tools: Viral vectors, primary cultures, microfluidic, MEA, rodent model, animal models & intracranial delivery. |
 | Dr. Sophie Halliez, PhD. Associate Professor – Chair of excellence Inserm. University of Lille, Faculty of Medicine. Teaching : Molecular and cell neurobiology. Research : Experimental models, physiological et pathological neural networks, cell mechanisms in tauopathies. Methods : Multielectrode arrays, microfluidics and neurobiology |
 | Dr. Malika Hamdane, PhD. Professor of Cell Biology (HDR-Docent). University of Lille, Faculty of Medicine. Teaching: Cell Biology, Biochemistry, Neuroscience. Research: “Truncated Tau species”. The role in Alzheimer’s Disease and therapeutic and diagnosis applications. |
 | Dr. Bruno Lefebvre, PhD. Associate Professor (HDR). University of Lille, Faculty of Medicine. Teaching: Biochemistry, molecular and cell biology, epigenetics. Research: Tau and nucleic acids. |
 | Dr. Didier Vieau, PhD. |
Associate Professors and Professors | |
 | Prof. Vincent Deramecourt, MD, PhD. professor of histology – Hospital Biologist. University of Lille, Faculty of Medicine. Teaching: : Anatomo-pathology, Histology, Dean's Assessor for PACES. Research : Biomarkers of dementias - Clinical correlation. Hospital activities: Neurologist - Neuropathologist. |
 | Dr. Claire-Marie Dhaenens, PharmD, PhD, HDR, MCU-PH University of Lille, Faculty of Medicine. Research : Involved in genetics and epigenomics of Tauopathies and neurodegenerative disorders. Investigation of the MAPT gene regulation (alternative splicing, alternative promoter usage, transcription…) to explain the differential expression of Tau proteins in human brain and in experimental models. |
 | Dr. Thibaud Lebouvier, MD, PhD. Associate Professor – Hospital Biologist. University of Lille, Faculty of Medicine. Research : Frontotemporal lobar degenerations. Hospital duties: Neurologist. |
 | Dr Susanna Schraen-Maschke, PharmD, PhD, HDR, MCU-PH University of Lille, Faculty of Medicine. Research: Involved in the translational development and evaluation of biomarkers of Alzheimer’s disease in human biological fluids. Samples come from french national cohorts of patients, and on samples of the Lille University Hospital care. The aim is to evaluate their interest for diagnosis and prognosis of dementia. Hospital duties : Biochemistry and Biomarkers of Dementias. |
 | Dr Vincent Huin, MD, PhD, MCU-PH University of Lille, Faculty of Medicine. Research : Epigénomique des maladies neurodégénératives. Maladies à expansions de triplets Hospital duties : Biochemistry and Genetics of Neurodegenerative Diseases. |
 | Dr Marie Dubar, M.D, Ph.D, MCU-PH Université de Lille, Faculté de Médecine. |
Research support staff | |
| Séverine Bégard IE, Inserm | |
| Thomas Bouillet Ingineer on contract | |
| Anaïs Poncet Hospital Ingineer on contract | |
| Emilie Faivre IGE, Univ | |
| Raphaëlle Caillierez IGE, Univ | |
| Sébastien Carrier Tech, Univ | |
| Sabiha Eddarkaoui IGE, Univ | |
| Thomas Comptdaer ASI, Univ | |
| Sarah Leclercq Ingineer on contract | |
| Camille Lefebvre Assistant Ingineer on contract | |
| Espérance Pastouret Tech, Inserm | |
| |
| Benderradji Hamza, MD, PhD | |
| Danis Clément, PhD | |
| Namasivayam Balasubramaniam, PhD | |
| Nebie Ouada, PhD | |
| Vijayashankara Jhenkruthi, PhD | |
| Adriana Figueroa-Garcia | |
| |
| Coku Ilda | |
| Gauvrit Thibaut | |
| Launay Agathe | |
| Oosterlynck Marie | |
| Regost Claire | |
| Taouili Rislane | |
Alumni
- SABLONNIERE Bernard, MD, PhD
- SAYED RASSUL Muhammed, PhD
- MARCHAND Antoine, PhD
- DENECHAUD Marine, PhD
- KRAIEM Sarra, PhD
- GUEDJDAL Sarah, PhD
- PERBET Romain, PhD
- TAUTOU Marie, PhD
- LEROUX Elodie, PhD
- RICO Thomas, PhD
- SOUZA Cristiano, PhD
- LEROY Mélanie, PhD
- ZEJNELI Orgeta, PhD
- BAUD Catherine, PhD
- CARVALHO Kevin, PhD
- GOMEZ-MURCIA Victoria, PhD
- MERIAUX Céline, PhD
- SCHIRMER Claire, PhD
- VINDTDEUX Valérie, PhD
- ALBERT Marie, PhD
- DANIS Clément, PhD
- ERDUAL Edmone, PhD
- EVRARD Caroline, PhD
- GILLES, Mélissa, PhD
- HOMA Mégane, PhD
- ALVES-PIRES Claire, PhD
- ANDO Kunie, PhD
- BELARBI Karim, PhD
- BOMBOIS Stéphanie, MD, PhD
- BRETTEVILLE Alexis, PhD
- BROUILLETTE Jonathan, PhD
- BURNOUF Sylvie, PhD
- BUSSIERE Thierry, PhD
- CAILLET-BOUDIN Marie-Laure, PhD
- CARPENTIER Céline, PhD
- CELLAI Lucrezia, PhD
- CHARTIER-HARLIN Marie-Christine, PhD
- CHAUFERLIER Alban, PhD
- DAVID Jean-Philippe, MD, PhD
- DELACOURTE André, PhD
- DELAY Charlotte, PhD
- DELOBEL Patrice, PhD
- DELPLANQUE Jérôme, PhD
- DERISBOURG Maxime, PhD
- DOURLEN Pierre, PhD
- DUJARDIN Simon, PhD
- DUPONT-WALLOIS Lætitia, PhD
- FERNANDEZ-GOMEZ Francisco J, PhD
- FERREIRA Stéphanie, PhD
- FERRETTI Roberta, PhD
- FLAMENT Stéphane, PhD
- GELE Patrick, PhD
- GHANEMGHANEM Dana, PhD
- GOMPEL Marie, PhD
- HUIN Vincent , MD, PhD
- HUMEZ Sandrine, PhD
- JUMEAU Fanny, PhD
- LAMBERT Jean-Charles, PhD
- LAURENT Cyril, PhD
- LEBOUCHER Antoine, PhD
- LECOLLE Katia, PhD
- LEFRANC Didier, PhD
- LE FRECHE Hélène, MD
- LEGHAY Coline, PhD
- LEROY Olivier, PhD
- MAILLIOT Christel, PhD
- MARCINIAK Élodie, PhD
- MARINANGELI Claudia, PhD
- MAURAGE Claude-Alain, MD, PhD
- PAPEGAEY Anthony, PhD
- PEREZ-TUR Jordi, PhD
- PERMANNE Bruno, PhD
- PODEVIN-DIMSTER Valérie, PhD
- SAMBO Anne-Véronique, PhD
- SAUTIERE Pierre-Éric, PhD
- SCHINDOWSKI Katharina, PhD
- SIGALA Julien, MD, PhD
- SOULIE Cathia, PhD
- SPOLCOVA Andrea, PhD
- SULTAN Audrey, PhD
- TARDIVEL-SAFI Meryem, PhD
- TRAN Hélène, PhD
- TROQUIER Lætitia, PhD
- VERMERSCH Patrick, MD, PhD
- VIOLET Marie, PhD
A. MAPT regulation: epigenomics, transcriptional and post-transcriptional
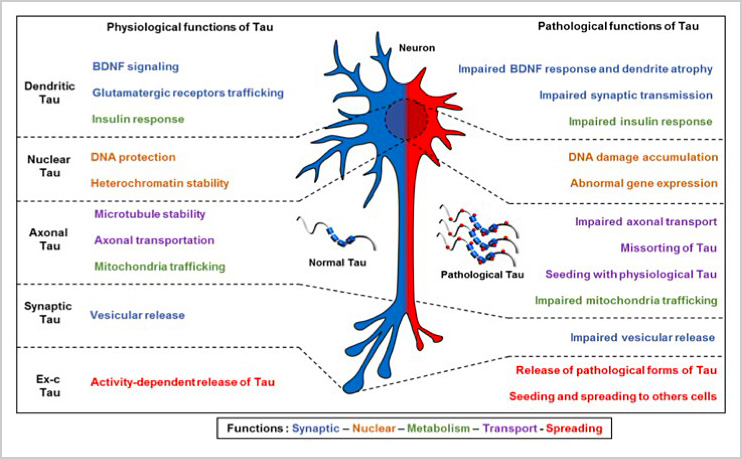
 B. New insights on Tau functions
B. New insights on Tau functions
Tau protein is originally defined as a microtubule-associated protein. Tau is also a signaling protein that clearly has other functions in the neuron and neuronal networks via membrane, nuclear and extracellular processes. These new functions can be carried by specific protein domains and / or post-translational modifications (phosphorylation, acetylation, proteolysis, etc.).
In this context, the laboratory is interested in (1) the role of Tau in the nucleus in particular in the protection of nucleic acids, the structure of chromatin and transcription; (2) the role of new amino-truncated forms of Tau in neuronal signaling and physiopathological development, particularly in patients; (3) the role of Tau as an extracellular signaling factor with the aim of understanding the mechanisms leading to its secretion under physiological and pathological conditions; (4) the role of Tau as a regulator of neuronal signaling.
 C. Modulating Tau pathology
C. Modulating Tau pathology
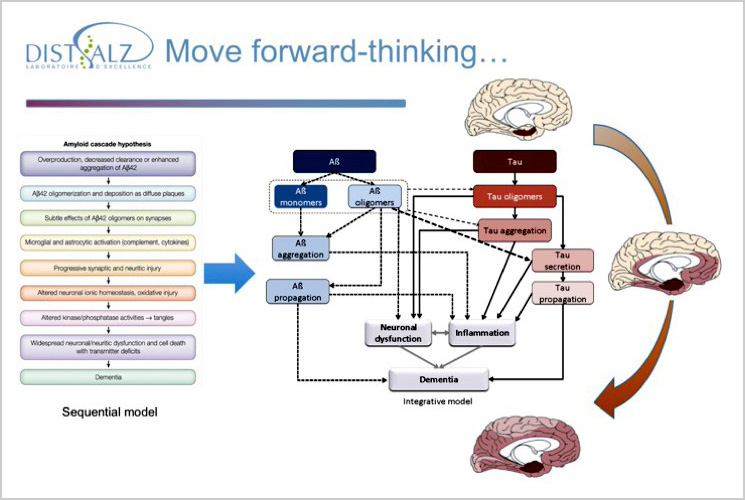
The laboratory is interested rôle pathogénique des différentes espèces moléculaire in the pathogenic role of the different molecular species of Tau especially in the prion-like spreading hypothesis. We are also interested in the role of extrinsic factors in the toxicity of these species with an interest for APP and the Tau-Aß interface, the role of potential pathological modulators resulting from proteomic / transcriptomic / genomic analyzes (GWAS with LabEx DISTALZ ), environmental factors (including caffeine and its targets, A2A adenosinergic receptors) and cellular factors (neurons vs. glial cells).
 D. Translational research: diagnostic and therapeutic applications
D. Translational research: diagnostic and therapeutic applications
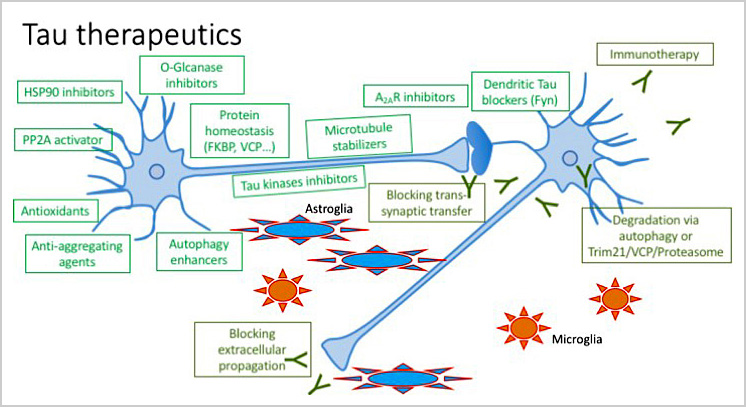
Our laboratory is committed to developing recherche translationnelle en interface avec les biologistes et clinicientranslational research in interface with biologists and clinicians. On one hand, we are interested in the identification of markers in autopsy-derived brain specimens and also body fluids (cerebrospinal fluid, blood…), allowing the differential diagnosis of dementia. We are developing a small cohort ECTAUSOME for a proof of concept. The emergence of new predictive biomarkers is essential for therapeutic implementation. On the other hand, we are developing preclinical research aimed at defining new therapeutic strategies. As such, we are exploring new anti-Tau compounds either by small molecule approaches (anti-aggregating, targeting protein chaperones…) in collaboration with the team of Patricia Melnyk – or immunotherapeutic approaches. The adenosinergic receptor pathway is also explored. These approaches are conducted in partnership with industry. It is notable that one of our molecules (MSBD) has led to the development of a company (AlzProtect) and a clinical trial. This molecule should move into phase 2 soon.
In conclusion, the strength of our laboratory welcoming researchers (biochemists, cell and molecular biologists, neurobiologists) and clinicians is to integrate fundamental, pre-clinical and clinical approaches to better understand the biology of Tau, its physiopathological role and the impact in terms of diagnosis and therapy.
• Publications
• Patents
• PhD Theses
Publications
2022
Denechaud M, Geurs S, Comptdaer T, Begard S, Garcia-Núñez A, Pechereau LA, Bouillet T, Vermeiren Y, de Deyn P, Perbet R, Deramecourt V, Vanderhaeghen M, Vanuytven S, Lefebvre B, Bogaert E, Deglon N, Voet T, Colin M, Buee L, Dermaut B*, Galas MC*. Nuclear tau confines oxidative stress-associated cycling neurons in S phase as a pro-survival mechanism in Alzheimer’s disease. Prog Neurobiol. In press
Manet C, Mansuroglu Z, Conquet L, Bortolin V, Comptdaer T, Segrt H, Menidjel R, Stadler N, Tian G, Herit F, Niedergang F, Souès S, Buée L, Galas MC*, Montagutelli X*, Bonnefoy E*. Zika virus infection of mature neurons from immunocompetent mice generates disease-associated microglia and a tauopathy-like phenotype in link with a delayed interferon beta response. J Neuroinflammation, in press
Launay A, Nebie O, Vijayashankara J, Lebouvier T, Buée L, Faivre E, Blum D. The role of adenosine A2A receptors in Alzheimer’s Disease and Tauopathies. Neuropharmacology, in press
Arandel L, Matloka M, Klein AF, Rau F, Sureau A, Ney M, Cordier A, Kondili M, Polay-Espinoza M, Naouar N, Ferry A, Lemaitre M, Begard S, Colin M, Lamarre C, Tran H, Buée L, Marie J, Sergeant N*, Furling D* (2022) Reversal of RNA toxicity in myotonic dystrophy via a decoy RNA-binding protein with high affinity for expanded CUG repeats. Nat Biomed Eng, 6(2):207-220.
Benderradji H, Vernotte E, Soto Ares G, Woillez JP, Perbet R, Karnoub MEA, Soudan B, Jannin A, Assaker R, Buee L, Prevot V, Maurage CA, Pigny P, Vantyghem MC, Merlen E, Cortet Rudelli C (2022) Efficacy of lanreotide 120 mg primary therapy on tumor shrinkage and ophthalmologic symptoms in acromegaly after one month. Clin Endocrinol (Oxf), 97(1):52-63.
Benderradji H, Kraiem S, Courty E, Eddarkaoui S, Bourouh C, Faivre E, Rolland L, Caron E, Besegher M, Oger F, Boschetti T, Carvalho K, Thiroux B, Gauvrit T, Nicolas E, Gomez-Murcia V, Bogdanova A, Bongiovanni A, Muhr-Tailleux A, Lancel S, Bantubungi K, Sergeant N, Annicotte JS, Buée L, Vieau D, Blum D, Buée-Scherrer V (2022) Impaired glucose homeostasis in a tau knock-in mouse model. Front Mol Neurosci, 15:841892
Bouillet T, Ciba M, Alves CL, Rodrigues FA, Thielemann C, Colin M, Buée L, Halliez S (2022) Revisiting tau involvement in complex neural network remodelling through analysis of extracellular neuronal activity exhibited by organotypic brain slice co-cultures. J Neural Eng, 19 066026.
Carracedo S, Briand-Amirat L, Dordas-Perpinyà M, Ramos Escuredo Y, Delcombel R, Sergeant N, Delehedde M. ProAKAP4 protein marker: Towards a functional approach to male fertility. Anim Reprod Sci. 2022 Sep 22;247:107074.
Chen SM, Hsu TC, Chew CH, Huang WT, Amanda C, Lin YF, Eddarkaoui S, Buee L, Chen CC (2022) Microtube Array Membrane Encapsulated Cell Therapy: a novel platform technology solution for treatment of Alzheimer’s disease (AD). Int J Mol Sci, 23(12):6855
Coku O, Mutez E, Eddarkaoui S, Carrier S, Marchand A, Deldycke C, Goveas L, Baille G, Tir M, Magnez R, Thuru X, Vermeersch G, Vandenberghe W, Buée L, Defebvre L, Sablonnière B, Chartier-Harlin MC, Taymans JM, Huin V (2022) Functional analyses of two novel LRRK2 pathogenic variants in familial Parkinson’s disease. Mov Disord, 37(8)1761-67.
Da Costa PJ, Hamdane M, Buée L, Martin F (2022) Tau mRNA Metabolism in Neurodegenerative Diseases: A Tangle Journey. Biomedecines 10(2): 241.
Danis C*, Dupré E*, Zejneli O*, Caillierez R, Arrial A, Bégard S, Mortelecque J, Eddarkaoui S, Loyens A, Cantrelle FX, Hanoulle X, Rain JC, Colin M, Buee L*, Landrieu I* (2022) Inhibition of Tau seeding by targeting Tau nucleation core within neurons with a single domain antibody fragment. Mol Ther, 30 (4):1484-1499
De Fisenne MA, Yilmaz Z, De Decker R, Suain V, Buée L, Ando K, Brion JP, Leroy K. Alzheimer PHF-tau aggregates do not spread tau pathology to the brain via the Retino-tectal projection after intraocular injection in male mouse models. Neurobiol Dis, in press
Delila L, Nebie O, Ngoc Le NT, Barro L, Chou ML, Wu YW, Watanabe N, Takahara M, Buée L, Blum D, Devos D, Burnouf T (2022) Neuroprotective activity of a virus-safe nanofiltered human platelet lysate depleted of extracellular vesicles in Parkinson’s disease and traumatic brain injury models. Bioeng Transl Med doi.org/10.1002/btm2.10360
Dewaeles E, Carvalho K, Fellah S, Sim J, Boukrout N, Caillierez R, Ramakrishnan H, Van der Hauwaert C, Vijaya Shankara J, Martin N, Massri N, Launay A, Folger JK, De Schutter C, Larrue R, Loison I, Goujon M, Jung M, Le Gras S, Gomez-Murcia V, Faivre E, Lemaire J, Garat A, Beauval N, Maboudou P, Gnemmi V, Gibier JB, Buee L, Abbadie C, Glowacki F, Pottier N, Perrais M, Cunha RA, Annicotte JS, Laumet G*, Blum D*, Cauffiez C*. Istradefylline (KW6002) protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin anti-tumor effects. J Clin Invest 132(22):e152924. doi: 10.1172/JCI152924.
Dordas-Perpinyà M, Sergeant N, Ruelle I, Bruyas JF, Charreaux F, Michaud S, Carracedo S, Catalán J, Miró J, Delehedde M, Briand-Amirat L. ProAKAP4 Semen Concentrations as a Valuable Marker Protein of Post-Thawed Semen Quality and Bull Fertility: A Retrospective Study. Vet Sci. 2022 May 6;9(5):224.
Gauvrit T, Benderradji H, Buée L, Blum D, Vieau D (2022) Early-Life Environment Influence on Late-Onset Alzheimer's Disease. Front Cell Dev Biol, 10:834661.
Gomez-Murcia V, Carvalho K, Thiroux B, Caillierez R, Besegher M, Sergeant N, Buée L, Faivre E, Blum D (2022) Impact of chronic doxycycline treatment in the APP/PS1 mouse model of Alzheimer's disease. Neuropharmacology, 209:108999
Hanon O, Vidal JS, Lehmann S, Bombois S, Allinquant A, Tréluyer JM, Abdoul H, Gelé P, Delmaire C, Blanc F, Mangin JF, Buée L, Touchon J, Hugon J, Vellas B, Galbrun E, Benetos A, Berrut G, Paillaud E, Wallon D, Castelnovo G , Volpe-Gillot L, Paccalin M, Robert PH, Godefroy O, Camus V, Belmin J, Vandel P, Novella JL, Duron E, Rigaud AS, Schraen-Maschke S, Gabelle A on behalf of the BALTAZAR study group. Plasma β-amyloid (Aβ) as a relevant prognosis biomarker for conversion to dementia in subjects with mild cognitive impairment. The BALTAZAR Alzheimers Dement, doi: 10.1002/alz.12613
Lam S, Hérard AS, Boluda S, Petit F, Eddarkaoui S, Cambon K, Picq JL, Buée L, Duyckaerts C, Haïk S, Dhenain M (2022) Pathological changes induced by Alzheimer’s brain inoculation in amyloid-beta plaque-bearing mice. Acta Neuropathol Commun, 10:112
Leroux E, Perbet R, Caillierez R, Richetin K, Lieger S, Espourteille J, Bouillet T, Begard S, Danis C, Loyens A, Toni N, Deglon N, Deramecourt V, Schraen-Maschke S, Buee L*, Colin M* (2022) Extracellular vesicles: major actors of heterogeneity in tau spreading among human tauopathies. Mol Ther, 30 (2)782-97
Manfredi-Lozano M, Leysen V, Adamo M, Paiva I, Rovera R, Pignat JM, Fatima Ezzahra Timzoura F, Candlish M, Eddarkaoui S, Samuel A. Malone SA, Silva MSB, Trova S, Imbernon M, Tena-Sempere M, Claret M, Paoloni-Giacobino A, Plassard D, Emmanuelle Paccou E, Vionnet N, Acierno J, Maceski A, Lutti A, Pfrieger F, Rasika S, Santoni F, Boehm U, Ciofi P, Buée L, Haddjeri N, Boutillier AL, Kuhle J,Messina A, Draganski B, Giacobini P, Pitteloud N, Prevot V (2022) GnRH replacement rescues cognition in Down Syndrome. Science, 377(6610):eabq4515
Mesangeau C, Carato P, Renault N, Coevoet M, Larchanché PE, Barczyk A, Buée L, Sergeant N, Melnyk P. Discovery of Compounds That Selectively Repress the Amyloidogenic Processing of the Amyloid Precursor Protein: Design, Synthesis and Pharmacological Evaluation of Diphenylpyrazoles. Int J Mol Sci. 2022 Oct 28;23(21):13111.
Nebie O, Buée L, Blum D, Burnouf T (2022) Can the administration of platelet trophic factors to the brain help treat neurological disorders? Cell Mol Life Sci, 79(7):379
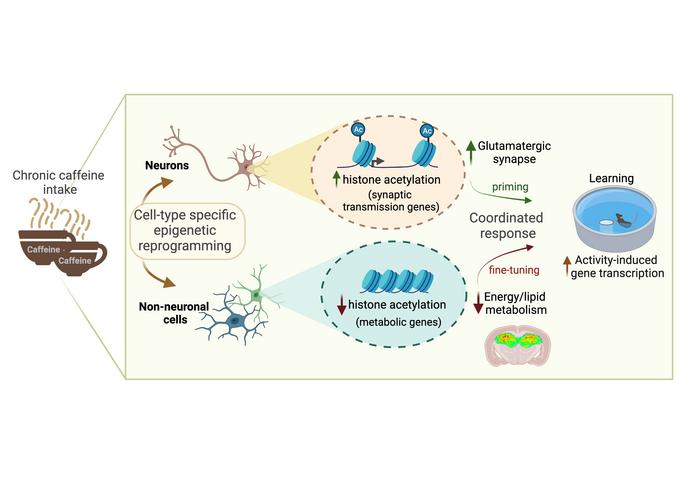
Paiva I, Cellai L, Mériaux C, Poncelet L, Papegaey A, Drobecq H, Le Gras S, Schneider M, Malik EM, Müller CE, Faivre E, Carvalho K, Gomez-Murcia V, Vieau D, Thiroux B, Eddarkaoui S, Lebouvier T, Schueller E, Seguin J, Stauber J, Lopes LV, Buée L, Buée-Scherrer V, Cunha RA, Ait-Belkacem R, Sergeant N, Annicotte JS, Boutillier AL, Blum D. Caffeine intake has a dual genome-wide effect on hippocampal metabolism and on learning-dependent transcription. J Clin Invest, 132(12):e149371
Petry S, Nateghi B, Keraudren R, Sergeant N, Planel E, Hébert SS, St-Amour I. Differential Regulation of Tau Exon 2 and 10 Isoforms in Huntington's Disease Brain. Neuroscience. 2022 Jul 19:S0306-4522(22)00367-0.
Rousset M, Humez S, Laurent C, Buée L, Blum D, Cens T, Vignes M, Charnet P (2022) Mammalian brain Ca2+ channel activity transplanted into Xenopus laevis oocytes. Membranes, 12(5)496.
Sexton C, Snyder H, Beher D, Boxer AL, Brannelly P, Brion JP, Buée L, Cacace AM, Chételat G, Citron M, DeVos SL, Diaz K, Feldman HH, Frost B, Goate AM, Gold M, Hyman BT, Johnson K, Karch CM, Kerwin DR, Koroshetz WJ, Litvan I, Morris HR, Mummery CJ, Mutamba J, Patterson MC, Quiroz YT, Rabinovici GD, Rommel A, Shulman MB, Toledo-Sherman LM, Weninger S, Wildsmith KR, Worley SL, Carrillo MC. Current directions in tau research: Highlights from Tau 2020. Alzheimers Dement, 2021, 1-20. https://doi.org/10.1002/alz.12452
Wang GS, Wang LH, Lin CY, Lin YM, Buée L, Sergeant N, Blum D, Chern Y (2022) Calpain-2 mediates MBNL2 degradation and a developmental RNA processing program in neurodegeneration. J Neurosci, 42:5102-14
Bertin E, Martinez A, Fayoux A, Carvalho K, Carracedo S, Fernagut PO, Koch-Nolte F, Blum D, Bertrand SS & Boué-Grabot E (2022) Increased surface P2X4 receptors by mutant SOD1 proteins contribute to ALS pathogenesis in SOD1-G93A mice. Cell Mol Life Sci 79(8):431.
Bantubungi K, Vieau D, Blum D*, Ferreira S* Uncovering bidirectional brain-body interactions in health and disease (2022) Neuropharmacology 5(1):91.
2021
Blum D, Lopes LV (2021) Stabilizing synapses. Science 374(6568):684-685. doi: 10.1126/science.abm3902
Brigas HC, Ribeiro M, Coelho JE, Gomes R, Gomez V, Carvalho K, Faivre E, Pereira S, Darrigues J, Antunes de Almeida A, Buée L, Dunot J, Marie H, Pousinha PA, Blum D, Silva-Santos B, Lopes LV, Ribot JC. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer's disease. Cell Reports, 36(9):109574
Carvalho K, Martin E, Cès A, Sarrazin N, Youssef I, Lagouge-Roussey P, Prigent A, Boluda S, Huin V, Fontaine B, Buée L, Delatour B, Dutar P, Sennlaub F, Guillonneau X, Blum D, Delarasse C. P2X7-deficiency rescues plasticity and cognitive deficits in a mouse model of Tauopathy. Prog Neurobiol, 206:102139
Chang CP, Chang YG, Chuang PY; Thi Ngoc Nguyen A; Wu KC, Chou FY; Cheng SJ, Chen HM, Jin LW, Carvalho K, Huin V, Buée L, Liao YF, Lin CJ, Blum D, Chern Y. ENT1 inhibition rescues energy dysfunction and pathology in a model of tauopathy. Acta Neuropathol Commun 9(1) 112
Damotte V, van der Lee SJ, Chouraki V, Grenier-Boley B, Simino J, Adams H, Tosto G, White C, Terzikhan N, Cruchaga C, Knol MJ, Li S, Schraen S, Grove ML, Satizabal C, Amin N, Berr C, Younkin S, Alzheimer’s Disease Neuroimaging Initiative, Gottesman RF, Buée L, Beiser A, Knopman DS, Uitterlinden A, DeCarli C, Bressler J, DeStefano A, Dartigues JF, Yang Q, Boerwinkle E, Tzourio C, Fornage M, Ikram MA, Amouyel P, de Jager P, Reitz C, Mosley Jr TH, Lambert JC, Seshadri S, Van Duijn C (2021) Plasma amyloid β levels are driven by genetic variants near APOE, BACE1, APP, PSEN2: A genome-wide association study in over 12,000 non-demented participants. Alzheimers Dement, 17(10):1663-1674
Hartnell IJ, Blum D, Nicoll JAR, Dorothee G, Boche D (2021) Glial cells and adaptive immunity in frontotemporal dementia with tau pathology. Brain. 144(3):724-745. doi: 10.1093/brain/awaa457. PMID: 33527991.
Lam S, Petit F, Hérard AS, Boluda S, Eddarkaoui S, Guillermier M, The Brainbank Neuro-CEB Neuropathology Network, Buée L, Duyckaerts C, Haïk S, Picq JL, Dhenain M (2021) Amyloid and tau pathologies, cognitive impairments and cerebral atrophy after Alzheimer brain extracts inoculation in a primate. Acta Neuropathol Commun, 9(1):165.
Lantoine J, Procès A, Villers A, Halliez S, Buée L, Ris L, Gabriele S (2021) Inflammatory molecules released by mechanically injured astrocytes trigger pre-synaptic loss in cortical neuronal networks. ACS Chem Neurosci, 12(20):3885-3897
Leroy M, Bertoux M, Skrobala E, Mode E, Adnet-Bonte C, Le Ber I, Bombois S, Cassagnaud P, Chen Y, Deramecourt V, Lebert F, Mackowiak MA, Sillaire AR, Wathelet M, Pasquier F, Lebouvier T; Méotis network (2021) Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimers Res Ther. 13(1):19. doi: 10.1186/s13195-020-00753-9.
Maté de Gérando A, d'Orange M, Augustin E, Joséphine C, Aurégan G, Gaudin-Guérif M, Guillermier M, Hérard AS, Stimmer L, Petit F, Gipchtein P, Jan C, Escartin C, Selingue E, Carvalho K, Blum D, Brouillet E, Hantraye P, Gaillard MC, Bonvento G, Bemelmans AP, Cambon K (2021) Neuronal tau species transfer to astrocytes and induce their loss according to tau aggregation state. Brain. 144(4):1167-1182. doi: 10.1093/brain/awab011.
Nebie O, Carvalho K, Barro L, Delila L, Faivre E, Renn TY, Chou ML, Wu YW, Nyem-Erdene A, Chou SY, Buée L, Hu CJ, Peng CW, Devos D, Blum D, Burnouf T (2021) Human platelet secretome brain administration for traumatic brain injury: pre-clinical assessment. Brain, 144(10) 3142-58.
Nebie O, Barro L, Wu YW, Knutson F, Buée L, Devos D, Peng CW, Blum D, Burnouf T (2021) Heat-treated human platelet lysate modulates microglia activation, favors wound healing and promotes neuronal differentiation in vitro. Platelets, 32(2): 226-37. doi: 10.1080/09537104.2020.1732324
Nyam-Erdene A, Nebie O, Delila L, Buée L, Devos D, Chou SY, Blum D, Burnouf T (2021) Characterization and Chromatographic Isolation of Platelet Extracellular Vesicles from Human Platelet Lysates for Applications in Neuroregenerative Medicine. ACS Biomater Sci Eng, 7(12): 5823-5835. doi: 10.1021/acsbiomaterials.1c01226.
Pietrowski MJ, Gabr AA, Kozlov S, Blum D, Halle A, Carvalho K (2021) Glial Purinergic Signaling in Neurodegeneration. Front Neurol. 12:654850. doi: 10.3389/fneur.2021.654850.
Rico T, Gilles M, Chauderlier A, Comptdaer T, Magnez R, Chwastyniak M, Drobecq H, Pinet F, Thuru X, Buee L, Galas MC, Lefebvre B (2021) Tau stabilizes chromatib compaction. Cell Dev. Biol - Cellular Biochemistry, 9:740550
Susloparova A, Halliez S, Begard S, Colin M, Buée L, Pecqueur S, Alibart F, Thomy V, Arscott S, Pallecchi E, Coffinier Y (2021) Low impedance and highly transparent microelectrode arrays (MEA) for in vitro neuron electrical activity probing. Sens Actuators B Chem, 327:128895
Tautou M, Eddarkaoui S, Descamps F, Larchanché PE, El Bakali J, Goveas LM, Dumoulin M, Lamarre C, Blum D, Buee L, Melnyk P, Sergeant N (2021) A β‐secretase modulator decreases Tau pathology and preserves short-term memory in a mouse model of neurofibrillary degeneration. Front Pharmacol, 12:679335.
Vaillant-Beuchot L, Mary A, Pardossi-Piquard R, Bourgeois A, Lauritzen I, Eysert F, Kinoshita PF, Cazareth J, Badot C, Fragaki K Bussiere R, Martin C, Mary R, Bauer C, Pagnotta S, Paquis-Flucklinger V, Buée-Scherrer V, Buée L, Lacas-Gervais S, Checler F, Chami M (2021) Amyloid precursor protein C-terminal fragments accumulation triggers mitochondrial structure, function and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol, 141(1): 39-65.
Vautheny A, Duwat C, Auregan G, Joséphine C, Hérard AS, Jan C, Mitja J, Gipchtein P, Gaillard MC, Buée L, Blum D, Hantraye P, Bonvento G, Brouillet E, Cambon K, Bemelmans AP (2021) THY-Tau22 mouse model accumulates more tauopathy at late stage of the disease in response to microglia deactivation through TREM2 deficiency. Neurobiol Dis, 155:105398. doi: 10.1016/j.nbd.2021.105398.
Wang ZH, Xia Y, Wu Z, Kang SS, Zhang JC, Liu P, Liu X, Song W, Huin V, Dhaenens CM, Yu SP, Wang XC, Ye K (2021) Neuronal ApoE4 stimulates C/EBPβ activation, promoting Alzheimer's disease pathology in a mouse model. Prog Neurobiol. 209:102212. doi: 10.1016/j.pneurobio.2021.102212.
2020
Ando K, De Decker R, Vergara C, Yilmaz Z, Mansour S, Suain V, Sleegers K, de Fisenne MA, Houben S, Potier MC, Duyckaerts C, Watanabe T, Buée L, Leroy K, Brion JP (2020) Picalm reduction exacerbates tau pathology in a murine tauopathy model. Acta Neuropathol, 139:773–89 doi:10.1007/s00401-020-02125-x
Ahmed T, Van Der Jeugd A, Caillierez R, Buee L, Blum D, D'Hooge R, Balschun D (2020) Chronic sodium selenate treatment restores deficits in cognition and synaptic plasticity in a murine model of tauopathy. Front Mol Neurosci, 13:570223. doi: 10.3389/fnmol.2020.570223
Bourefis AR, Campanari ML, Buee-Scherrer V, Kabashi E. Functional characterization of a FUS mutant zebrafish line as a novel genetic model for ALS. Neurobiol Dis. 142:104935. doi: 10.1016/j.nbd.2020.104935.
Colin M, Dujardin S, Schraen-Maschke S, Meno-Tetang G, Duyckaerts C, Courade JP, Buée L (2020) Exploring anti-tau immunotherapy within the hypothesis of the prion-like propagation. Acta Neuropathol, 139: 3-25
Degiorgis L, Karatas M, Sourty M, Faivre E, Lamy J, Noblet V, Bienert T, Reisert M, von Elverfeldt D, Buée L, Blum D, Boutillier AL, Armspach JP, Blanc F, Harsan LA (2020) Remodeling of cerebral networks architecture anticipate behavioral deficits in a tauopathy mouse model of Alzheimer's disease. Brain, 143(12):3748-3762. doi: 10.1093/brain/awaa312.
Duriez P, Eddarkaoui S, Blum D, Dickson SL, Gorwood P, Tolle V, Viltart O (2020) Does physical activity associated with chronic food restriction alleviate anxiety like behaviour, in female mice? Horm Behav. 124:104807. doi: 10.1016/j.yhbeh.2020.104807.
Gomez-Murcia V, Sandau U, Ferry B, Parrot S, Laurent C, Basquin M, Buée L, Boison D, Blum D (2020) Hyperexcitability and seizures in the THY-Tau22 mouse model of Tauopathy. Neurobiol Aging, 94: 265-270.
Hector A, McAnulty C, Piché-Lemieux ME, Alves-Pires C, Buée-Scherrer V, Buée L*, Brouillette J* (2020) Tau hyperphosphorylation induced by the anesthetic agent ketamine- xylazine involved the calmodulin-dependent protein kinase II. FASEB J, 34(2):2968–77
Homa M, Loyens A, Eddarkaoui S, Faivre E, Deramecourt V, Maurage CA, Buée L, Huin V, Sablonnière B (2020) The TMEM240 Protein, Mutated in SCA21, Is Expressed in Purkinje Cells and Synaptic T Cerebellum 19(3):358‐369. doi:10.1007/s12311-020-01112-y
Richetin K, Steullet P, Pachoud M, Perbet R, Parietti E, Maheswaran M, Eddarkaoui S, Bégard S, Pythoud C, Rey M, Caillierez R, Do KQ, Halliez S, Bezzi P, Buee L, Leuba G, Colin M, Toni N, Déglon N (2020) Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nature Neurosci, 23(12): 1567-79. https://doi.org/10.1038/s41593-020-00728-x
Runhart EH, Khan M, Cornelis SS, Roosing S, Del Pozo-Valero M, Lamey TM, Liskova P, Roberts L, Stöhr H, Klaver CCW, Hoyng CB, Cremers FPM, Dhaenens CM; Disease Consortium Study Group (2020) Association of Sex With Frequent and Mild ABCA4 Alleles in Stargardt Disease. JAMA Ophthalmol. 138(10):1035-1042. doi: 10.1001/jamaophthalmol.2020.2990.
Sierksma A, Lu A, Salta E, Mancuso R, Zoco J, Blum D, Buée L, De Strooper B, Fiers M (2020) Novel Alzheimer risk genes determine the microglia response to amyloid ß but not to TAU pathology. EMBO Mol Med, 12(3): e10606.
Temido-Ferreira M, Ferreira DG, Batalha VL, Marques-Morgado I, Coelho JE, Pereira P, Gomes R, Carvalho S, Canas PM, Cuvelier L, Buée-Scherrer V, Faivre E, CE, Pimentel J, Schiffmann SN, Buée L, Outeiro TF, Bader M, Blum D, Cunha RA, Marie H, Pousinha PA, Lopes LV (2020) Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol Psychiatr, 25(8):1876-1900
Verelst J, Geukens N, Eddarkaoui S, Vliegen D, De Smidt E, Rosseels J, Franssens V, Molenberghs S, Francois C, Stoops E, Bjerke M, Engelborghs S, Laghmouchi M, Carmans S, Buée L, Vanmechelen E, Winderickx J, Thomas D (2020) A Novel Tau Antibody Detecting the First Amino-Terminal Insert Reveals Conformational Differences Among Tau Isoforms. Front Mol Biosci, 7:48. doi:10.3389/fmolb.2020.00048
Zheng J, Akbari M, Schirmer C, Reynaert ML, Loyens A, Lefebvre B, Buée L, Croteau DL, Galas MC, Bohr VA (2020) Hippocampal tau oligomerization early in tau pathology coincides with a transient alteration of mitochondrial homeostasis and DNA repair in a mouse model of tauopathy. Acta Neuropathol Commun, 8(1):25. doi:10.1186/s40478-020-00896-8
2019
Albert M, Mairet-Coello G, Danis C, Lieger S, Caillierez R, Carrier S, Skrobala E, Landrieu I, Michel A, Schmitt M, Citron M, Downey P, Courade JP, Buée L*, Colin M* (2019) Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain, 142(6): 1736-1750
Carvalho K, Faivre E, Pietrowski MJ, Marques X, Gomez-Murcia V, Deleau A, Huin V, Hansen JN, Kozlov S, Danis C, Temido-Ferreira M, Coelho JE, Mériaux C, Eddarkaoui S, Le Gras S, Dumoulin M Cellai L, NeuroCEB Brain Bank, Landrieu I, Chern Y, Hamdane M, Buée L, Boutillier AL, Levi S, Halle A, Lopes LV, Blum D (2019) Exacerbation of C1q dysregulation, synaptic loss and memory deficits in Tau pathology linked to neuronal adenosine A2A receptors. Brain, 142(11): 3636-54
Corvol JC, Buée L (2019) A new step towards targeting tau. Lancet Neurol, 18:517-8.
Domise M, Sauvé F, Didier S, Caillerez R, Bégard S, Carrier S, Colin M, Marinangeli C, Buee L, Vingtdeux V (2019) Neuronal AMP-activated protein kinase (AMPK) hyper-activation induces synaptic loss by an autophagy mediated process. Cell Death Dis, 10:221
Dupré E, Danis C, Arrial A, Cantrelle FX, Merzougui H, Hanoulle X, Colin M, Rain JC, Buée L*, Landrieu I* (2019) Single chain antibody fragments as new tools for studying the neuronal Tau protein physiopathology. ACS Chemical Neurosci, 10(9):3997-4006
Faldini E, Ahmed T, Buee L, Blum D, Balschun D (2019) Tau- but not Aß -pathology enhances NMDAR-dependent depotentiation in AD-mouse models. Acta Neuropathol Comm, 7:202
Fichou Y, Al-Hilaly YK, François Devred F, Smet-Nocca C Tsvetkov PO, Verelst J, Winderickx J, Geukens N, Vanmechelen E, Perrotin A, Serpell L, Hanseeuw B, Medina M, Buée L*, Landrieu I* (2019) The elusive Tau Molecular structures: Can we translate the recent breakthroughs into new targets for intervention? Acta Neuropathol Commun, 7:31
Gary C, Lam S, Herard AS, Koch JE, Petit F, Gipchtein P, Sawiak SJ, Caillierez R, Eddarkaoui S, Colin M, Aujard F, Deslys JP, French neuropathology network, Brouillet E, Buée L, Comoy EE, Pifferi F, Picq JL, Dhenain M (2019) Encephalopathy induced by Alzheimer brain inoculation in a non-human primate. Acta Neuropathol Commun, 7(1)126.
Houben S, Leroy K, Ando K, Yilmaz Z, Widomski C, Buée L, Brion JP (2019) Genetic ablation of tau in postnatal neurons rescues decreased adult hippocampal neurogenesis in a tauopathy model. Neurobiol Dis, 127:131-141
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus R, Tejera D, Griep A, Santarelli F, Brosseron F, Opitz S, Stunden J, Merten M, Kayed R, Golenbock D, Blum D, Latz E, Buee L, Heneka M (2019) NLRP3 inflammasome activation drives tau pathology. Nature, 575(7784): 669-673
Jadhav S, Avila J, Schöll M, Kovacs G, Kövari E, Skrabana R, Evans L, Kontsekova E, Malawska B, de Silva R, Buée L*, Zilka N* (2019) A walk through tau therapeutic strategies. Acta Neuropathol Commun, 7: 22.
Leboucher A, Ahmed T, Caron E, Tailleux A, Raison S, Joly Amado A, Marciniak E, Carvalho K, Hamdane M, Bantubungi K, Lancel S, Eddarkaoui S, Caillierez R, Vallez E, Staels B, Vieau D, Balschun D, Buée L*, Blum D* (2019) Brain insulin response and peripheral metabolic changes in a Tau transgenic mouse model. Neurobiol Dis, 125 :14-22.
Lemaire Q, Raffo-Romero A, Arab T, Van Camp C, Drago F, Forte S, Gimeno JP, Begard S, Colin M, Vizioli J, Sautière PE, Salzet M, Lefebvre C (2019) Isolation of microglia-derived extracellular vesicles: towards miRNA signatures and neuroprotection. J Nanobiotechnology. 17(1):119. doi: 10.1186/s12951-019-0551-6.
Loera-Valencia R, Cedazo-Minguez A, Kenigsberg PA, Page G, Duarte A, Giusti P, Zusso M, Robert P, Frisoni G, Cattaneo A, Zille M, Boltze J, Cartier N, Buee L, Johansson G, Winblad B (2019) Current and emerging avenues for Alzheimer’s disease drug targets. J Intern Med, 286(4):398-437
Nebie O, Devos D, Vingtdeux V, Barro L, Devedjian JC, Jonneaux A, Chou ML, Bordet R, Buée L, Knutson F, Blum D, Burnouf T (2019) The neuroprotective activity of heat-treated human platelet lysate biomaterials manufactured from outdated pathogen-reduced (amotosalen/UVA) platelet concentrates. J Biomed Sci, 26(1):89. doi: 10.1186/s12929-019-0579-9
Paiva I, Carvalho K, Cellai L, Santos P, Pavlou MAS, Jain G, Gnad T, Pfeifer A, Vieau D, Fischer A, Buée L, Outeiro TF, Blum D (2019) A2AR-induced transcriptional deregulation in astrocytes: an in vitro Glia, 67(12):2329-2342
Sergeant N, Vingtdeux V, Eddarkaoui S, Gay M, Le Fur N, Evrard C, Laurent C, Caillierez R, Obriot H, Larchanché PE, Farce A, Coevoet M, Carato P, Kouach M, Descat A, Dallemagne P, Buée-Scherrer V, Hamdane M, Buee L, Melnyk P (2019) New piperazine multi-effect drugs prevent neurofibrillary degeneration and amyloid deposition, and preserve memory in animal models of Alzheimer’s disease. Neurobiol Dis, 129: 217-33.
Shrivastava AN, Redeker V, Pieri L, Bousset L, Renner M, Madiona K, Mailhes-Hamon C, Coens A, Buée L, Hantraye P, Triller A, Melki R (2019) Clustering of tau fibrils impairs the synaptic composition of a3-Na+/K+-ATPase and AMPA receptors. EMBO J, 38, e99871
Zappettini S, Faivre E, Ghestem A, Carrier S, Buée L, Blum D, Esclapez M, Bernard C (2019) Caffeine Consumption During Pregnancy Accelerates the Development of Cognitive Deficits in Offspring in a Model of Tauopathy. Front Cell Neurosci; 13: 438. doi: 10.3389/fncel.2019.00438.
Patents
Patented
- EP17210486.1 Cauffiez C, Perrais M, Blum D. KIT-OF-PARTS COMPRISING A NEPHROTOXIC CHEMOTHERAPEUTIC DRUG AND A SELECTIVE A2A ADENOSINE RECEPTOR ANTAGONIST. December 22, 2017.
- EP17305355.4 Hamdane M, Derisbourg M, Leghay C, Chiappetta G, Verdier Y, Vinh J, Blum D, Buée L. New Tau species. March 28, 2017.
Patents accepted
- WO2015068075 – Buée L, Colin M, Hantraye P, Aron Badin R, Dujardin S, Bemelmans A, Brouillet E. DETECTION OF TAU.
- WO2014096321 – Buée L, Troquier L, Lassalle P. ANTIBODIES SPECIFIC TO TAU PHOSPHORYLATED AT SERINE 422 AND USES FOR THE TREATMENT AND DIAGNOSIS OF TAUOPATHIES.
Licenced Patents
- US20160356785 Sergeant N, Mitchell V, Jumeau F, Sigala J. Methods for determining human sperm quality. Licence exclusive à 4BioDx.
- WO2016146655 : Sergeant N, Melnyk P, Buée L. Novel 1,4-bis(3-aminopropyl)piperazine derivatives and its use. 16 mars 2015 (Accord de licence en discussion avec la société Alzprotect).
- WO2015032842 : Sergeant N, Mitchell V, Jumeau F, Sigala J. Méthodes de détermination de la qualité du sperme humain. Licence exclusive à 4BioDx.
- WO2014102339 : Barrier M, Buée L, Burlet S, Delacourte A, Estrella C, Melnyk P, Sergeant N, Verwaerde P. Sulfate salts of N-(3-(4-(3-(diisobutylamino)propyl)piperazin-1-yl)propyl)-1H-benzo[d]imidazol-2-amine. AlzProtecT – phase I terminée en 2015.
- W02006051489 : Melnyk Patricia, Sergeant Nicolas, Buée Luc, Delacourte André, Inserm, Université de Lille II, « Use of 1, 4-bis(3 aminopropyl)piperazine derivatives in therapy ». http://www.wipo.int/pctdb/en/wo.jsp?WO=2006051489 Licensed to AlzProtecT.
Molécule actuellement en essai clinique chez l’homme – phase I terminée en 2015.
Phd Theses
Ongoing Phd
- CARVALHO Kevin (D2 en 2017 – thesis director : David BLUM)
- DENECHAUD Marine (D1 en 2018 – thesis director : Marie-Christine GALAS)
- ERDUAL Edmone (D3 en 2017 – thesis director : Christelle CAUFFIEZ et David BLUM)
- GUEDJDAL Sarah (D1 en 2017 – thesis director : Malika HAMDANE)
- GUIRAUD Sebastien (D3 en 2018 – thesis director : MC GALAS/R PARDOSSI)
- HOMA Megane (D2 en 2017 – thesis director : Bernard SABLONNIERE)
- NEBIE Ouada (D1 en 2017 – thesis director : David BLUM et Thierry BURNOUF)
- LEROUX Elodie (D1 en 2018 – thesis director : Morvane COLIN)
- LEROY Melanie (D1 en 2018 – thesis director : Vincent DERAMECOURT)
- PERBET Romain (D1 en 2017 – thesis director : Morvane COLIN)
- RICO Thomas (D1 en 2018 – thesis director : Bruno LEFEBVRE)
- SOUSA Cristiano (D1 en 2018 – thesis director : Malika HAMDANE et Karen VANHOORELBEKE)
- TAUTOU Marie (D1 en 2018 – thesis director : Nicolas SERGEANT)
- ZEJNELI Orgeta (D1 en 2018 – thesis director : Luc BUEE et Isabelle LANDRIEU)
- ALBERT Marie (sout. le 10/12/2018 directed by Morvane COLIN) (title/abstract)
- DANIS Clément (soutenance le 14/12/2018 directed by Isabelle LANDRIEU et Luc BUEE) (title/abstract)
- DOMISE Manon (soutenance le 17/12/2018 directed by Valerie VINGTDEUX) (title/abstract)
- EVRARD Caroline (sout. le 20/11/2018 directed by Nicolas SERGEANT) (title/abstract)
- GILLES Melissa (sout. le 13/11/2018 directed by Bruno LEFEBVRE) (title/abstract)
- TARDIVEL-SAFI Meryem (sout. le 6/12/2017 directed by Morvane COLIN) (title/abstract)
- LEGHAY Coline (sout. le 19/09/2017 directed by Malika HAMDANE (title/abstract)
- PAPEGAEY Anthony (sout. le 19/12/2016) directed by Valerie BUEE-SCHERRER (title/abstract)
- CHAUDERLIER Alban (sout. le 16/12/2016) directed by Marie-Christine GALAS (title/abstract)
- HUIN Vincent (sout. le 15/12/2016) directed by Bernard SABLONNIERE et Claire-Marie DHAENENS (title/abstract)
- SIGALA Julien (sout. le 08/12/2016) directed by Valérie MITCHELL et Nicolas SERGEANT (title/abstract)
- MARCINIAK Elodie (sout. le 14/12/2015) directed by Sandrine HUMEZ (title/abstract)
- ALVES-PIRES Claire (sout. le 10/12/2015) directed by Luc BUEE et Christelle MONACA-CHARLEY (title/abstract)
- SPOLCOVA Andrea (sout. le 23/09/2015) directed by Marie-Christine GALAS (title/abstract)
- DUJARDIN Simon (sout. le 21/09/2015) directed by Morvane COLIN (title/abstract)
- DERISBOURG Maxime (sout. le 30/03/2015) directed by Malika HAMDANE (title/abstract)
- VIOLET Marie (sout. le 28/02/2014) directed by Marie-Christine GALAS (title/abstract)
- LAURENT Cyril (sout. le 17/12/2013) directed by David BLUM (title/abstract)
- CARPENTIER Céline (sout. le 16/12/2013)directed by Marie-Laure CAILLET-BOUDIN (title/abstract)
- WATTEZ Jean-sébastien (sout. le 09/12/2013) directed by Didier VIEAU et Benoit FOLIGNE (title/abstract)
- JUMEAU Fanny (sout. le 16/09/2013) directed by Valérie MITCHELL et Nicolas SERGEANT (title/abstract)