Dr. Sowmyalakshmi Rasika, Ph.D.,
Contract Researcher ; WATCH project

Located on the campus of the CHU de Lille, the main objective of our laboratory is to decipher the complex dialogue that takes place between the brain and the rest of the body: the role played by the hypothalamus in the control of metabolism and reproduction, and the consequences of a deregulation of this dialogue on the body’s homeostasis, cognitive functions and brain aging. Our laboratory has an international dimension, bringing together the skills of neuroscientists and physicians specialized in reproductive and metabolic disorders and neurosurgery. We work in permanent collaboration with numerous research teams around the world.
Vincent Prévot, Président de Commission scientifique FNRS et Directeur de recherches à l’INSERM, “à propos de la liberté de chercher et de l’importance de l’expérimentation animale pour la recherche scientifique“, Soirée fondamentale du FNRS, 25 octobre 2023
An Enduring Dream of Science: Q&A with Vincent Prevot, PhD
Vincent Prévot, winner of the 2023 Grand Prize of the Foundation for Medical Research

Read the article
The new therapy that could change Eliaou’s life
New Avenues to Reduce Long-Term Complications in Preterm Infants
A Therapy Found to Improve Cognitive Function in Patients with Down Syndrome
Portrait of Inserm
Adrian Coutteau Robles
A “mini puberty” in babies?

The miniNO project could relieve tens of millions of lives.
Podcast available on Euroradio or live on Sound Cloud

Laurine Decoster wins the 1st public prize
The WATCH project elected “success story” by the European Commission.
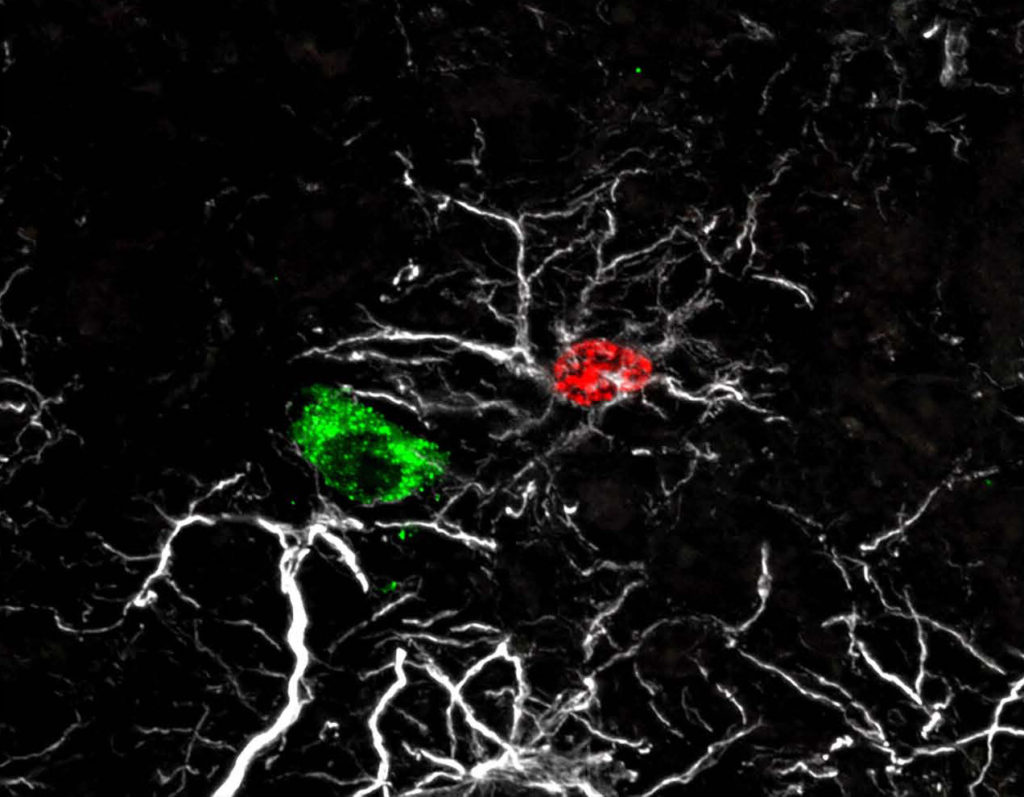
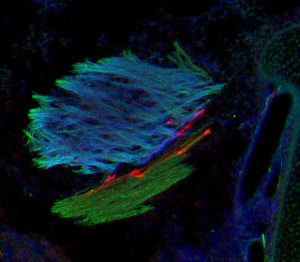
Neural mechanism facilitates
network integration for fertility-controlling neurons and sexual maturation.
How the Covid attacks the brain.
European researchers believe they have uncovered the mechanism by which the virus destroys the walls of the blood vessels that supply neurons, which would explain some neurological disorders.
LE FIGARO 17th november 2021
(pdf article)



“La Voix du Nord” (March 13, 2022)
Researchers track the effects of Covid on fertility and aging

“Les Echos” (September 13, 2021)
New light on diabetes
Inserm News (02 Août 2021)
Inserm News (08 fév 2021) Transmission of PCOS from mother to daughter: the epigenetics involved |



Down Syndrome, a hope of treatment.
The Health Mag.
Therapy improves cognitive functions
Young talent prize awarded to Nour El Houda Mimouni by the L’Oréal Foundation and UNESCO for Women and Science.
The nose, gateway to the Covid: what dangers in the neuronal development of the child? SDS 2021
Health challenge
“The VERTEXA project”.
What if aging well depended on the gateway for hormones to the brain??
Projet WATCH for Well-Aging and the Tanycytic Control of Health.
Sensation of satiety: chronicle of Thierry Lhermitte
Vincent Prevot presents his symposium “Hypothalamic tanycytes in metabolic regulation”.
 | LAB DIRECTOR Vincent Prevot’s research focuses on Systems Neuroscience and Neuroendocrinology, in particular the brain circuits that control reproduction and metabolism and the neural pathways through which they respond to peripheral information. |
 | Dr. Paolo Giacobini, Ph.D. Expert on developmental neuroscience and neuronal migration. |
 | Dr. Sébastien Bouret, Ph.D. Expert in the neuroendocrinology of obesity. Sebastien's general research interest is to study the role of the perinatal environment and neurodevelopmental mechanisms in lifelong obesity and metabolism.Find out more about Sebastien's work. |
 | Dr Virginie Mattot, Ph.D. Expertise in : Vascular biology (angiogenesis, endothelial activation, endothelial cell functions and properties) and vascular extracellular matrix. |
 | Dr Alicia Mayeuf-Louchart, Ph.D. Expert in developmental biology and metabolism of skeletal muscle and brown adipose tissue. Alicia's research focuses on understanding the formation of brown adipose tissue and skeletal muscle during development and the perinatal period, as well as their circadian and metabolic regulation. |
 | Dr. Ines Martinez Corral Ph.D. |
 | Dr. Ariane Sharif, Ph.D. Ariane’s Research focuses on the importance of glial cells in regulating neuroendocrine processes and targeting therapeutic interventions for glioblastomas. |
 | Dr. Bénédicte Dehouck, Ph.D. Study of blood-brain interfaces in contributing to the delivery of blood-borne molecules to their neuronal targets. |
 | Dr. Marc Baroncini, M.D, Ph.D. Marc is a neurosurgeon, hospital practitioner at the University Hospital of Lille. He is interested in the anatomy of the hypothalamus, hypothalamic control of metabolism and deregulation in eating disorders. He is also involved in the surgical pathology of cerebrospinal fluid. |
 | Dr Vincent Florent, M.D, Ph.D. Vincent is a hospital physician specializing in clinical nutrition, metabolism and eating disorders. He is conducting several translational studies including the study of the communication of the hypothalamus with the periphery in humans. He has designed an innovative virtual reality therapeutic game program called VERTEXA, aimed at treating eating disorders. |
 | Dr. Konstantina Chachlaki, Ph.D. Contract Researcher; miniNO project |
 |
 | Dr. Sophie Catteau-Jonard, PU-PH, M.D, Ph.D. Principal Research Areas: Ovulation disorders including polycystic ovary syndrome, hypothalamic amenorrhea, hypogonadic hypogonadism and premature ovarian failure. |
 | Pr. Claude-Alain Maurage, M.D., Ph.D. |
 | Dr. Anne-Laure Barbotin, M.D, Ph.D. Neurostructural hypothalamic plasticity in polycystic ovary syndrome.Formation : Master 2, Paris V University, France. |
 | Dr. Cécile Allet, Ph.D. Studies : Doctoral thesis of the University of Lille "Gliogenesis in the hypothalamus during postnatal development: implication in the control of female sexual maturation". |
 | Dr. Émilie Caron, Ph.D.
|
 | Sarah Gallet
Impact of melatonin hormone on the blood-brain-barrier at the level of the median eminence. Studies : M.Sc. Biology Biotechnology, University of Lille, 2011. |
 | Manon Leclerc |
 | Shengyue Deng |
 | Maxime Delit |
 | Danielle Mazur
Responsibilities: Maintenance and utilization of the cryostats, microtomes etc. Perfusion Room. |
 | Amandine Legrand
|
 | Dr. Betty Rodriguez-Cortez, Ph.D |
 | Dr. Daniela Fernandois, Ph.D |
 | Dr. Sreekala Nampoothiri, Ph.D |
 | Dr. Ludovica Cotellessa, Ph.D
|
 | Dr. Marialetizia Rastelli, Ph.D
|
 | Dr. Gaetan Ternier, Ph.D
|
 | Dr. Florent Sauvé, Ph.D |
 | Dr. Sooraj NAIR D.M.V, Ph.D |
 | Virginia Delli
|
 | Eleonora Deligia
|
 | Rémi Kurdian
|
 | Mouna Accary
|
 | Amine Bouchekioua |
 | Fatima Timzoura
|
 | Pierre-Yves Barelle
|
 | Ophélie Hannot
|
 | Alicia Sicardi
|
 | Nabil Nasri
|
 | Amine Belfoul
|
 | Claire Jasinki |
 | Mathilde Roux |
 | Rachel Spenle |
 | Ricardo Martinez-Gomez |
 | Sixtine Karmann |
 | Loïc Kacimi |
 | Charles-Antoine Seux |
 | Caio Coelho, PhD |
 | Laurine Decoster, PhD |
 | Dr. Laura Kuczynski, Ph.D |
 | Dr. Monica Imbernon, Ph.D |
 | Dr. Adrian Coutteau Robles, Ph.D |
 | Dr. Nour El Houda Mimouni, Ph.D |
 | Dr. Mauro Silva, Ph.D |
 | Dr. Marion Martin, Ph.D |
 | Dr. Maëliss Peigné, M.D, Ph.D
|
 | Dr. Manon Duquenne, Ph.D |
 | Dr. Sophie Crozier, Ph.D Group Leader at the UNIL, Lausanne, Switzerland https://www.unil.ch/cig/en/home/menuinst/research/dr-sophie-croizier.html |
 | Dr. Valerie Leysen, Ph.D |
 | Dr. Charlotte Vanacker, Ph.D Post Doctoral Research Fellow, University of Michigan. Moenter Lab, Molecular & Integrative Physiology Department. |
 | Dr. Irène Cimino, Ph.D Post Doctoral Research Fellow, University of Cambridge. Yeo Lab, Metabolic Research. |
 | Dr. Mathieu Méquinion, Ph.D Post Doctoral Fellow, University of Monash, Zane Lab, Australia. |
 | Dr. Gustav Colldén, Ph.D Post Doctoral Research Fellow, German Research Centre for Environmental Health (GmbH). Institute for Diabetes and Obesity. Tschöp Lab. |
 | Dr. Jyoti Parkash, Ph.D Assistant Professor Central University of Punjab, Bhatinda, India. |
 | Dr. Andrea Messina, Ph.D Research Manager, University Hospital of Lausanne, Switzerland, Pitteloud Lab. |
 | Dr. Filippo Casoni, Ph.D Assistant Professor Universita Vita-Salute Sane Raffaele Milan. |
 | Dr. Julien Mallard, Ph.D Ph.D Student |
 | Dr. Fanny Langlet, Ph.D
|
 | Dr. Églantine Balland, Ph.D |
 | Dr. Naresh Hanchate, Ph.D |
 | Dr. Nicole Bellefontaine, Ph.D |
 | Dr. Sophie Steculorum, Ph.D
|
 | Dr. Xavier d’Anglemont de Tassigny, Ph.D |
 | Dr. Amandine Mullier, Ph.D |
 | Dr. Sara Trova, Ph.D |
 | Dr. Giuliana Pellegrino, Ph.D |
 | Dr. Samuel Malone, Ph.D Postdoctoral Research Scientist, Department of Physiology, Anatomy and Genetics, Domingos’ Lab, University of Oxford |
 | Dr. Sonal Shruti, Ph.D Upstream Data Coordinator, UCB Pharma, Belgium. |
 | Dr. Jérôme Clasadonte, Ph.D Senior Scientist Epilepsy, Team Leader at UCB BioPharma, Belgium. |
 | Dr. Maria Manfredi Lozano, Ph.D Postdoctoral Fellow, Instituto de Biomedicina de Sevilla, D’anglemont de Tassigny’s Lab, Spain. |
It also aims to evaluate how pathological conditions (obesity, diabetes etc.) affect these neurobiological events and, conversely, how the impairment of communication between the brain and the periphery renders the organism prone to developing such conditions (obesity, diabetes, precocious and/or delayed puberty and infertility). Our laboratory brings together in a single setting a wide range of theoretical knowledge and technical expertise in its fields of research and is able to investigate physiological and pathological processes from the molecular and genetic/epigenetic levels to that of human patients, thanks to the close interaction between basic scientists and clinicians. For the excellence of our work, our laboratory has been classified as “remarkable” (the highest “grade” attributed to French research laboratories) by our evaluating bodies in 2014 and 2019.
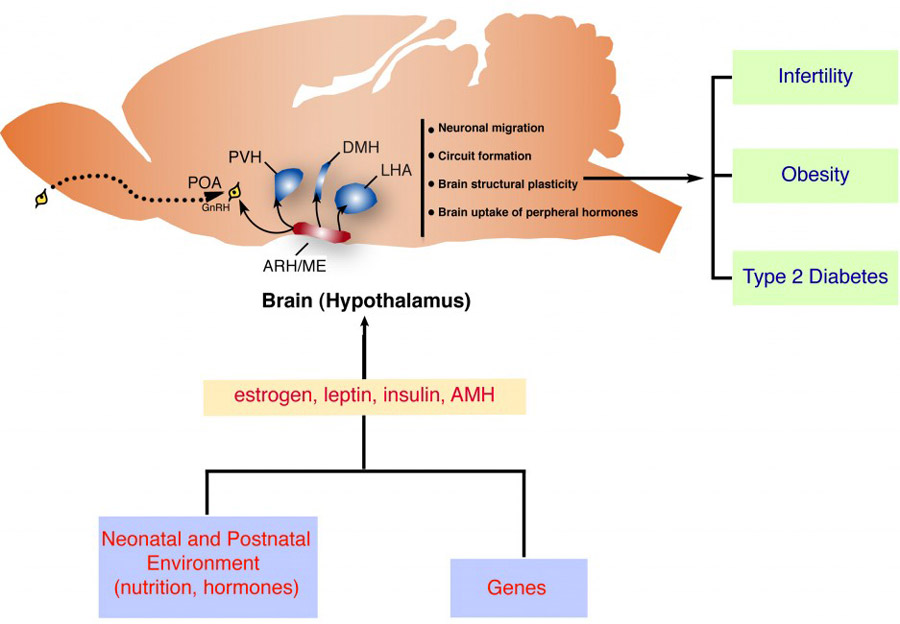 Abbreviations: ARH/me: arcuate nucleus of the hypothalamus/médian eminence; GnRH: gonadotropin releasing hormone; POA: preoptic region; PVH: Periventricular nucleus of the hypothalamus; DMH: dorsomedial nucleus of the hypothalamus; LHA: lateral hypothalamic area; AMH: anti-Mullerian hormone.
Our Lab is also actively involved in several Local (FHU 1000 days for Health, DN2M), National (Société de Neuroendocrinologie (SNE), GDR repro, Société des neurosciences (http://www.neurosciences.asso.fr), Club de cellules gliales) and International research networks including LARC-Neurosciences, GnRH network, and FENS.
Abbreviations: ARH/me: arcuate nucleus of the hypothalamus/médian eminence; GnRH: gonadotropin releasing hormone; POA: preoptic region; PVH: Periventricular nucleus of the hypothalamus; DMH: dorsomedial nucleus of the hypothalamus; LHA: lateral hypothalamic area; AMH: anti-Mullerian hormone.
Our Lab is also actively involved in several Local (FHU 1000 days for Health, DN2M), National (Société de Neuroendocrinologie (SNE), GDR repro, Société des neurosciences (http://www.neurosciences.asso.fr), Club de cellules gliales) and International research networks including LARC-Neurosciences, GnRH network, and FENS.
2023
Hypothalamic neuroglial plasticity is regulated by anti-Müllerian hormone and disrupted in polycystic ovary syndrome.
Barbotin AL, Mimouni NEH, Kuchcinski G, Lopes R, Viard R, Rasika S, Mazur D, Silva MSB, Simon V, Boursier A, Pruvo JP, Yu Q, Candlish M, Boehm U, Bello FD, Medana C, Pigny P, Dewailly D, Prevot V, Catteau-Jonard S, Giacobini P.EBioMedicine. 2023;90:104535. doi: 10.1016/j.ebiom.2023.104535PMID: 37001236
Overactivation of GnRH neurons is sufficient to trigger polycystic ovary syndrome-like traits in female mice.
Silva MSB, Decoster L, Delpouve G, Lhomme T, Ternier G, Prevot V, Giacobini P.
EBioMedicine. 2023;97:104850. doi: 10.1016/j.ebiom.2023.104850.PMID: 37898094
Maternal low-calorie sweetener consumption rewires hypothalamic melanocortin circuits via a gut microbial co-metabolite pathway.
Park S, Belfoul AM, Rastelli M, Jang A, Monnoye M, Bae H, Kamitakahara A, Giavalisco P, Sun S, Barelle PY, Plows J, Jang C, Fodor A, Goran MI, Bouret SG.JCI Insight. 2023;8(10):e156397. doi: 10.1172/jci.insight.156397.PMID: 37014702
Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death.
Sauve F, Nampoothiri S, Clarke SA, Fernandois D, Ferreira Coêlho CF, Dewisme J, Mills EG, Ternier G, Cotellessa L, Iglesias-Garcia C, Mueller-Fielitz H, Lebouvier T, Perbet R, Florent V, Baroncini M, Sharif A, Ereño-Orbea J, Mercado-Gómez M, Palazon A, Mattot V, Pasquier F, Catteau-Jonard S, Martinez-Chantar M, Hrabovszky E, Jourdain M, Deplanque D, Morelli A, Guarnieri G, Storme L, Robil C, Trottein F, Nogueiras R, Schwaninger M, Pigny P, Poissy J, Chachlaki K, Maurage CA, Giacobini P, Dhillo W, Rasika S, Prevot V.
EBioMedicine. 2023;96:104784. doi: 10.1016/j.ebiom.2023.104784. PMID: 37713808
Protocol for simultaneous patch-clamp recording from tanycytes and neurons in living mouse brain slices.
Lhomme T, Prévot V.
STAR Protoc. 2023 20;4(4):102571. doi: 10.1016/j.xpro.2023.102571. PMID: 37733593
COVID-19 could worsen cerebral amyloid angiopathy.
Dewisme J, Lebouvier T, Vannod-Michel Q; Lille COVID Research Network (LICORNE); Prevot V, Maurage CA.
J Neuropathol Exp Neurol. 2023 21;82(9):814-817. doi: 10.1093/jnen/nlad049.PMID: 37428895
Cell proliferation and glial cell marker expression in the wall of the third ventricle in the tuberal region of the male mouse hypothalamus during postnatal development.
Coutteau-Robles A, Prevot V, Sharif A.
J Neuroendocrinol. 2023;35(3):e13239. doi: 10.1111/jne.13239. PMID: 36863859
Male minipuberty involves the gonad-independent activation of preoptic nNOS neurons.
Delli V, Dehame J, Franssen D, Rasika S, Parent AS, Prevot V, Chachlaki K.
Free Radic Biol Med. 2023 Jan;194:199-208. doi: 10.1016/j.freeradbiomed.2022.11.040. PMID: 36470319
2022
NOS1 mutations cause hypogonadotropic hypogonadism with sensory and cognitive deficits that can be reversed in infantile mice.
Female sexual behavior is disrupted in a preclinical mouse model of PCOS via an attenuated hypothalamic nitric oxide pathway.
Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions.
Melatonin drugs inhibit SARS-CoV-2 entry into the brain and virus-induced damage of cerebral small vessels.
Cecon E, Fernandois D, Renault N, Coelho CFF, Wenzel J, Bedart C, Izabelle C, Gallet S, Le Poder S, Klonjkowski B, Schwaninger M, Prevot V, Dam J, Jockers R. Cell Mol Life Sci. 2022 Jun 13;79(7):361. doi: 10.1007/s00018-022-04390-3.
2021
The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells.
GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation.
Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function.
Duquenne M, Folgueira C, Bourouh C, Millet M, Silva A, Clasadonte J, Imbernon M, Fernandois D, Martinez-Corral I, Kusumakshi S, Caron E, Rasika S, Deliglia E, Jouy N, Oishi A, Mazzone M, Trinquet E, Tavernier J, Kim YB, Ory S, Jockers, R., Schwaninger, M., Boehm, U., Nogueiras, R., Annicotte, J.S., Gasman, S., Dam, J. & Prevot, V.
Nat Metab 2021. 3: 1071-1090
Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons.
Lhomme T, Clasadonte J, Imbernon M, Fernandois D, Sauve F, Caron E, Lima N, Heras V, Martinez-Corral I, Muller-Fielitz H, Rasika S, Schwaninger M, Nogueiras R, Prevot V.
J Clin Invest 2021. 131:e140521.10.1172/JCI140521
Mutations in SEMA3F and PLXNA3 Encoding Semaphorin-3F and its Receptor Plexin-A3 Respectively Cause Idiopathic Hypogonadotropic Hypogonadism.
Kotan, L.D., Ternier, G., Cakir, A.D., Emeksiz, H.C., Turan, I., Delpouve, G., Kardelen, A.D., Ozcabi, B., Isik, E., Mengen, E., Cakir, E.D.P., Yuksel, A., Agladioglu, S.Y., Dilek, S.O., Evliyaoglu, O., Darendeliler, F., Gurbuz, F., Akkus, G., Yuksel, B., Giacobini, P. & Topaloglu, A.K. (2021) Loss-of-Function Genetics in Medicine.
Genet Med 2021. 23/1008-1016. DOI: 10.1038/s41436-020-01087-5
Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process.
Mimouni NEH, Paiva I, Barbotin AL, Timzoura FE, Plassard D, Le Gras S, Ternier G, Pigny P, Catteau-Jonard S, Simon V, Prevot V, Boutillier AL, Giacobini P (2021) Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab 33:513-530.e8 DOI: 10.1016/j.cmet.2021.01.004
2020
The proportion of cleaved anti-Müllerian hormone is higher in serum but not follicular fluid of obese women independently of polycystic ovary syndrome.
DOI: Peigné M., Pigny1 P., Pankhurst M.W., Drumez E., Loyens A., Dewailly D., Catteau-Jonard S., Giacobini P.
Reproductive BioMedicine Online 2020 , 41:1112-1121. DOI: 10.1016/j.rbmo.2020.07.020.
Neuron-Derived Neurotrophic Factor Is Mutated in Congenital Hypogonadotropic Hypogonadism.
Messina A, Pulli K, Santini S, Acierno J, Känsäkoski J, Cassatella D, Xu C, Casoni F, Malone SA, Ternier G, Conte D, Sidis Y, Tommiska J, Vaaralahti K, Dwyer A, Gothilf Y, Merlo GR, Santoni F, Niederländer NJ, Giacobini P*, Raivio T*, Pitteloud N*
Am J Hum Genet. 2020, 106:58-70.* Equal contribution.
Neuropilin-1 expression in GnRH neurons regulates prepubertal weight gain and sexual attraction.
Vanacker C, Trova S, Shruti S, Casoni F, Messina A, Croizier S, Malone S, Ternier G, Hanchate NK, Bouret SG, Ciofi P, Giacobini P, Prevot V
Embo J 2020. 39, e104633
The endoplasmic reticulum stress-autophagy pathway controls hypothalamic development and energy balance regulation in leptin-deficient neonates.
Park S, Aintablian A, Coupe B, Bouret SG
Nat Commun 2020. 11, 1914
Maternal obesity-induced endoplasmic reticulum stress causes metabolic alterations and abnormal hypothalamic development in the offspring.
Park S, Jang A, Bouret SG
PLoS Biol 2020. 18, e3000296
Hypothalamic Structural and Functional Imbalances in Anorexia Nervosa.
Florent V, Baroncini M, Jissendi-Tchofo P, Lopes R, Vanhoutte M, Rasika S, Pruvo JP, Vignau J, Verdun S, Johansen JE, Pigeyre M, Bouret SG, Nilsson IAK, Prevot V.
Neuroendocrinology. 2020. 110, 552-562.
2019
Human Semaphorin 3 Variants Link Melanocortin Circuit Development and Energy Balance.
Van der Klaauw AA, Croizier S, Mendes de Oliveira E, Stadler LKJ, Park S, Kong Y, Banton MC, Tandon P, Hendricks AE, Keogh JM, Riley SE, Papadia S, Henning E, Bounds R, Bochukova EG, Mistry V, O'Rahilly S, Simerly RB, Consortium UK, , Minchin, J.E.N., Barroso, I., Jones, E.Y., Bouret, S.G. & Farooqi, I.S.
Cell 2019. 176: 729-742 e18
Non-nutritive Sweeteners Induce Hypothalamic ER Stress Causing Abnormal Axon Outgrowth.
Park, S., Sethi, S. & Bouret, S.G.
Front Endocrinol (Lausanne) 2019. 10, 876.
Defective AMH signaling disrupts GnRH neuron development and function and contributes to hypogonadotropic hypogonadism.
Malone, S.A., Papadakis, G.E., Messina, A., Mimouni, N.E.H., Trova, S., Imbernon, M., Allet, C., Cimino, I., Acierno, J., Cassatella, D., Xu, C., Quinton, R., Szinnai, G., Pigny, P., Alonso-Cotchico, L., Masgrau, L., Marechal, J.D., Prevot, V., Pitteloud, N. & Giacobini, P.
Elife 2019. 8: e47198. doi: 10.7554/eLife.47198
2018
Central Dicer-miR-103/107 controls developmental switch of POMC progenitors into NPY neurons and impacts glucose homeostasis.
Croizier, S., Park, S., Maillard, J. & Bouret, S.G.
Elife 2018. 7: e40429
Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood
Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P.
Nat Med. 2018. doi: 10.1038/s41591-018-0035-5.
A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and grey mouse lemur (Microcebus murinus)
Pellegrino G, Trubert C, Terrien J, Pifferi F, Leroy D, Loyens A, Migaud M, Baronicini M, Maurage CA, Fontaine C, Prevot V, Sharif A
J Comp Neurol. 2018. 526(9):1419-1443
2017
Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle
Clasadonte, J., E. Scemes, Z. Wang, D. Boison, and P.G. Haydon.
Neuron. 2017. 95:1365-1380.e5.
Phenotyping of nNOS Neurons in the Postnatal and Adult Female Mouse Hypothalamus
Chachlaki K, Malone SA, Qualls-Creekmore, Hrabovszky E, Munzberg H, Giacobini P, Ango F, Prevot V
J Comp Neurol, 2017. 525:3177-3189
Tridimensional Visualization and Analysis of Early Human Development
Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, Chedotal A
Cell. 2017. 169:161-173 e112
2023
New Horizons: Gonadotropin-Releasing Hormone and Cognition.
Prévot V, Tena-Sempere M, Pitteloud N.
J Clin Endocrinol Metab. 2023;108(11):2747-2758. doi: 10.1210/clinem/dgad319.PMID: 37261390 Review.
Food odour recognition adjusts systemic metabolism to nutrient availability.
Prevot V, Nogueiras R.
Nat Rev Endocrinol. 2023;19(3):130-131. doi: 10.1038/s41574-022-00801-4.PMID: 36599948 No abstract available.
2022
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis.
Sowing SARS-CoV-2 to reap neurodegeneration: A hamster study.
The polygamous GnRH neuron: Astrocytic and tanycytic communication with a neuroendocrine neuronal population.
Glycemic control: Tanycytes march to the beat of the suprachiasmatic drummer.
Glial control of neuronal function.
Developmental programming of hypothalamic melanocortin circuits.
Molecular control of the development of hypothalamic neurons involved in metabolic regulation.
2021
Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology Sharif A, Fitzsimons CP, Lucassen PJ. Handb Clin Neurol. 2021;179:125-140.
Tanycytes in the infundibular nucleus and median eminence and their role in the blood-brain barrier.
Prevot, V., Nogueiras, R. & Schwaninger, M.
Handb Clin Neurol 2021, 180: 253-273.
The KiNG of reproduction: Kisspeptin/ nNOS interactions shaping hypothalamic GnRH release.
Delli, V., Silva, M.S.B., Prevot, V. & Chachlaki, K.
Mol Cell Endocrinol 2021, 532:111302.
New insights into anti-Mullerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development.
Silva, M.S.B. & Giacobini, P.
Cell Mol Life Sci. 2021, 78:1-16. DOI: 10.1007/s00018-020-03576-x.78: 1-16.
A roadmap for the Human Developmental Cell Atlas.
Haniffa, M., Taylor, D., Linnarsson, S., Aronow, B.J., Bader, G.D., Barker, R.A., Camara, P.G., Camp, J.G., Chedotal, A., Copp, A., Etchevers, H.C., Giacobini, P., Gottgens, B., Guo, G., Hupalowska, A., James, K.R., Kirby, E., Kriegstein, A., Lundeberg, J., Marioni, J.C., Meyer, K.B., Niakan, K.K., Nilsson, M., Olabi, B., Pe'er, D., Regev, A., Rood, J., Rozenblatt-Rosen, O., Satija, R., Teichmann, S.A., Treutlein, B., Vento-Tormo, R., Webb, S. & Human Cell Atlas Developmental Biological
Nature 2021. 597: 196-205.
Unveiling the Importance of Tanycytes in the Control of the Dialogue Between the Brain and the Periphery.
Nampoothiri S, Duquenne M, Prevot V.
In: Tasker JG, et al., ed. Glial-Neuronal Signaling in Neuroendocrine Systems.
Masterclass in Neuroendocrinology Series. Switzerland: Springer Nature:255-284, 2021
2020
Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome.
Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, Abbott DH.
Endocr Rev. 2020. 41,538-76.
Nitric oxide signalling in the brain and its control of bodily functions.
Chachlaki K, Prevot V.
Br J Pharmacol. 2020,177:5437-5458.
2019
Emerging Roles of Anti-Müllerian Hormone in Hypothalamic-Pituitary Function.
Barbotin AL, Peigné M, Malone SA, Giacobini P.
Neuroendocrinology. 2019. 109,218-229.
Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism.
García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH.
Nat Neurosci. 2019. 22,7-14.
Don’t Trust Your Gut: When Gut Microbiota Disrupt Fertility.
Silva MSB, Giacobini P.
Cell Metab. 2019, 30:616-618. doi: 10.1016/j.cmet.2019.09.005.
New Developments in Reproductive and Stress Neuroendocrinology.
Prevot V, Millar RP.
Neuroendocrinology. 2019, 109:191-192. doi: 10.1159/000502420.
2018
Wired for eating: how is an active feeding circuitry established in the postnatal brain?
Muscatelli F, Bouret SG.
Curr Opin Neurobiol. 2018. 52, 165-171.
Sex and gender differences in developmental programming of metabolism.
Dearden L, Bouret SG, Ozanne SE.
Mol Metab. 2018.15, 8-19.
The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus
Clasadonte J & Prevot V
Nature Reviews Endocrinology. 2018, 14:25-44.
The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism
Prevot, V., B. Dehouck, A. Sharif, P. Ciofi, P. Giacobini, and J. Clasadonte.
Endocrine Reviews. 2018. doi: 10.1210/er.2017-00235.
2017
The gentle art of saying NO: how nitric oxide gets things done in the hypothalamus
Chachlaki K, Garthwaite J, Prevot V
Nature Reviews Endocrinology. 2017, 13:521-535.
When size matters: how astrocytic processes shape metabolism
Sharif A, Prevot V
Cell Metabolism. 2017, 25:995-996.
2023
Minipuberty: A critical period for brain functional development.
Chachlaki K, Le Duc K, Storme L, Prévot V.
Med Sci (Paris). 2023;39(10):697-700. doi: 10.1051/medsci/2023113..PMID: 37943124 French. No abstract available.
Down syndrome, GnRH and cognition.
Prévot V, Pitteloud N.
Med Sci (Paris). 2023;39(4):316-318. doi: 10.1051/medsci/2023037..PMID: 37094260 French. No abstract available.
2021
Precocious puberty and neuropilin-1 signaling in GnRH neurons.
Vanacker C, Bouret SG, Giacobini P, Prévot V.
Med Sci (Paris). 2021 Apr;37(4):366-371. doi: 10.1051/medsci/2021035. Epub 2021 Apr 28.
2018
Rôle des réseaux astrocytaires métaboliques dans le maintien de l’éveil
Clasadonte J
Med Sci (Paris) 2017; 34(3):199-202
2017
Les micro-ARN Nouveaux acteurs du contrôle hypothalamique de la fertilité
Messina A, Langlet F, Prevot V
Med Sci (Paris) 2017; 33:506-511
Tanycytes hypothalamiques, barrière hématoencéphalique et rôle dans la régulation de l’homéostasie énergétique
Florent V, Baroncini M, Prevot V
Cahiers de Nutrition et de Diététique, 52)1):26-32
Développement des neurones à GnRH dans le cerveau d’embryons humains
Barbotin AL, Prevot V, Giacobini P
Medi Sci (Pari) 2017; 33:376-379
